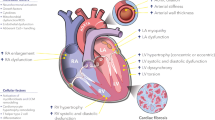
Abstract
This review evaluates the role of mechanotransduction (MT) in heart failure (HF) pathobiology. Cardiac functional and structural modifications are regulated by biomechanical forces. Exposing cardiomyocytes and the myocardial tissue to altered biomechanical stress precipitates changes in the end-diastolic wall stress (EDWS). Thereby various interconnected biomolecular pathways, essentially mediated and orchestrated by MT, are launched and jointly contribute to adapt and remodel the myocardium. This cardiac MT-mediated feedback decisively determines the primary cardiac cellular and tissue response, the sort (concentric or eccentric) of hypertrophy/remodeling, to mechanical and/or hemodynamic alterations. Moreover, the altered EDWS affects the diastolic myocardial properties independent of the systolic function, and elevated EDWS causes diastolic dysfunction. The close interconnection between MT pathways and the cell nucleus, the genetic endowment, principally allows for the wide variety of phenotypic appearances. However, demographic, environmental features, comorbidities, and also the genetic make-up may modulate the phenotypic result. Cardiac MT takes a fundamental and superordinate position in the myocardial adaptation and remodeling processes in all HF categories and phenotypes. Therefore, the effects of MT should be integrated in all our scientific, clinical, and therapeutic considerations.



Similar content being viewed by others
Notes
Fibrotic tissue replaces specific myocardial tissue (replacement fibrosis) if myocardial tissue is lost (notably if necrotic) such as in case of myocardial infarction, myocarditis, etc. [Herum K (2017) J Clin Invest 6, 53; doi:https://doi.org/10.3390/jcm6050053]. Reactive myocardial fibrosis occurs as an accompanying process in case of hypertrophic (eccentric and concentric) remodeling [Weber KT (2013) Nat Rev. Cardiol 10: 15–26; Herum K (2017) J Clin Invest 6, 53; doi:https://doi.org/10.3390/jcm6050053]. However, there is a very strong interconnection and coupling between any inflammatory activity and pro-fibrotic pathways in response to any threat the organism is exposed to [Chen L (2018) Oncotarget 9: 7204–7218; Chen L (2017) Nat Immunol 18: 825].
The term remodeling describes all cardiac molecular, cellular, tissue, and geometrical changes displayed in response to any bio-physical stress the heart is exposed to, which collectively form and determine the modified, adapted heart structure and function [Omens JJ (2007). In: Cardiac mechanotransduction by Tavi and Weckstroem. New York: Springer Science and Business Media, chapter 5, pp. 78–92; Cohn JN (2000) J Am Coll Cardiol 35: 596–582].
Myocardial stiffness, basically referring to “material properties” of the cardiac tissue (namely the cardiomyocytes and of the extracellular matrix) [Chaturvedi RR et al. (2010) Circulation 121: 979–988], and chamber stiffness need to be thoroughly distinguished [Gaasch WH et al. (1982) Eur Heart J 3 (Suppl A): A 139 – A 145].
Natriuretic peptides take adaptive measures by promoting natriuresis and diuresis, inhibiting the sympathetic nervous system and renin-angiotensin-aldosterone activity, and display an arterial vasodilatory effect [Adams jr KF (2003) Am Heart J 145: S 34–S 46]. As such, natriuretic peptides counteract the neurohumoral systems.
BNP is a load-indiced cardiomyocyte specific marker of modified gene expression [Meluzin J, Tomandl, J (2015) Hindawi Volume 2015, Article ID 426045, 9 pages].
References
Savarese GI, Lund LH (2017) Global public health burden of heart failure. Cardiac Failure Review 3:7–11
Mensah GA, Wei GS, Sorlie PD et al (2017) Decline in cardiovascular mortality: possible causes and implications. Circ Res 120:366–380
Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray J, Rahimi K (2018) Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 391:572–580
Damman K, Tang WH, Felker GM, Lassus J, Zannad F, Krum H, McMurray J (2014) Current evidence on treatment of patients with chronic systolic heart failure and renal insufficiency: practical considerations from published data. J Am Coll Cardiol 63:853–871
Konstam MA, Abboud FM (2017) Ejection fraction: misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 135:717–719
Najjar SS (2009) Heart failure with preserved ejection fraction: failure to preserve, failure of reserve, and failure on the compliance curve. J Am Coll Cardiol 54:419–421
Reichek N, Wilson J, St. John M et al (1982) Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation 65:99–108
Ponikowsky P, Voors AA, Anker SD et al (2016) 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 37:2129–2200
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey de Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride P, McMurray J, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 128:1810–1852
Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-la Rocca HP, TIME-CHF investigators (2015) Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs reduced left vebtricular ejection fraction. Eur J Heart Fail 17:1006–1014
Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen D, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA (2018) Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 72:1081–1090
Zile MR, Baicu CF (2013) Biomarkers of diastolic dysfunction and myocardial fibrosis: application to heart failure with a preserved ejection fraction. J Cardiovasc Transl Res 6:501–515
Borlaug BA, Redfield MM (2011) Diastolic and systolic heart failure are distinct phenotypes of the heart failure syndrome. Circulation 123:2006–2014
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271
Borlaug BA (2014) The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 11:507–515
Van Heerebeek L, Borbély A, Niessen HW et al (2006) Myocxardial structure and function differ in systolic and diastolic heart failure. Circulation 113:1966–1973
Rodrigues PG, Leite-Moreira AF, Falcão-Pires I (2016) Myocardial reverse remodeling: how far can we rewind Am J Physiol heart Circ Physiol 310: H 1402 - H 1422
Van Linthout S, Tschoepe C (2017) Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep 14:251–265
Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, de Keulenaer GW (2019) The continous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 40:2155–2163
Toischer K, Rokita AG, Unsöld B, Zhu W, Kararigas G, Sossalla S, Reuter SP, Becker A, Teucher N, Seidler T, Grebe C, Preuss L, Gupta SN, Schmidt K, Lehnart SE, Krüger M, Linke WA, Backs J, Regitz-Zagrosek V, Schäfer K, Field LJ, Maier LS, Hasenfuss G (2010) Differential cardiac remodeling in preload versus afterload. Circulation 122:993–1003
Swynghedaue B (2016) Evolutionary paradigms in cardiology: the case of chronic heart failure. In: Alvergne S, Jenkins C, Fauri C (eds) Evolutionary thinking in medicine. Advances in the Evolutionary Analysis of Human Behaviour. Springer Intern Publishing, Switzerland, pp 137–153
Ingber DE (2003) Mechanobiology and diseases of machanotransduction. Ann Med 35:1–14
Orr AW, Helmke BP, Blackman BR, Schwartz MA (2006) Mechanisms of mechanotransduction. Dev Cell 10:11–20
Ingber DE (1997) The architectural basis of cellular mechanotransduction. Annu Rev Physiol 59:575–599
Voorhees AP, Han H-C (2016) Biomechanics of cardiac function. Compr Physiol 5:1623–1644
Herum KM, Lunde IG, McCulloch AD, Christensen G (2017) The soft- and hard-heartedness of cardiac fibroblasts: Mechanotransduction signaling pathways in fibrosis of the heart. J Clin Med 6:53
Knöll R, Hoshijima M, Chien K (2003) Cardiac mechanotransduction and implications for heart disease. J Mol Med 81:750–756
Maksuti E, Westerhof BE, Ugander M, Donker DW, Carlsson M, Broomé M (2019) Cardiac remodeling in aortic and mitral valve disease - a simulation study with clinical validation. J Appl Physiol 126:1377–1389
Kim KH, Kim HM, Park JS, Kim YJ (2019) Differential Transcriptome profile and exercise capacity in cardiac remodeling by pressure overload versus volume overload. J Cardiovasc Imaging 27:50–63
Grossman W, Jones D, McLaurin LP (1975) Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 56:56–64
Gerdes AM, Campbell SE, Hilbelink DR (1988) Structural remodelling of cardiac myocytes in rats with arteriovenous fistula. Lab Investig 59:857–861
Mann DL, Bogaev R, Buckberg GD (2010) Cardiac remodelling and myocardial recovery: lost in translation? Eur J Heart Fail 12:789–796
Hill JA, Olson EN (2008) Cardiac plasticity. N Engl J Med 358:1370–1380
Vega ER, Konhilas JP, Kelly DP, Leinwand LA (2017) Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metabol 25:1012–1026
Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown P, Schocken DD (1992) Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation 86:426–430
Gori M, Iacovoni A, Senni M (2016) Hemodynamics of heart failure with preserved ejection fraction: a clinical perspective. Cardiac Fail Rev 2:102–105
Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN (2009) Regulatory T cells ameliorate angiotensin II–induced cardiac damage. Circulation 119:2904–2912
Anaversa P, Ricci R, Olivetti G (1986) Quantitative structural analysis of the myocardium during physiological growth and induced cardiac hypertrophy: a review. J Am Coll Cardiol 7:1140–1149
Tavi P, Laine M, Weckström M, Ruskoaho H (2001) Cardiac mechanotransduction: from sensing to disease and treatment. Trend Phram Sci 55:254–260
Grossman W, Paulus WJ (2013) Myocardial stress and hypertrophy: a complex interface between biophysics and cardiac remodeling. J Clin Invest 123:3701–3703
Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, Vargiu P, Simongini I, Laragh JH (1992) Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 19:1550–1558
Nadruz W (2015) Myocardial remodeling in hypertension. J Hum Hypertens 29:1–6
Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350:1953–1959
Chien KR (1999) Stress pathways and heart failure. Cell 98:555–558
Omens JH, McCulloch AD, Lorenzen-Schmidt I (2007) Mechanotransduction in cardiac remodeling and heart failure. In: Weckström M, Tavi P cardiac mehanotransduction. Springer Science and Business Media, New York, chapter 5:78–92
Hunter JJ, Chien KR (1999) Signaling pathways for cardiac hypertrophy and failure. N Engl J Med 341:1276–1283
Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H (2006) B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 47:742–748
Bansal M, Marwick TH (2008) Natriuretic peptides and filling pressure at rest and stress. Heart Fail Clin 4:71–86
Burkhoff D, Mirsky I, Suga H (2005) Assessment of systolic and diastolic ventricular properties via pressurevolume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H 501 - H 512
Fukuta H, Little WC (2008) The cardiac cycle and the pathophysiological basis of left ventricular contraction, ejection, relaxation, and filling. Heart Fail Clin 4:1–11
Kerkhof PLM (2015) Characterizing heart failure in the ventricular volume domain. Clin Med Insights Cardiol 9(Suppl 1):11–31
Mihl C, Dassen WRM, Kuipers H (2008) Cardiac remodeling versus eccentric hypertrophy in strength and endurance athletes. Neth Heart J 16:129–133
Beisvag V, Kemi OJ, Arbo I (2009) Pathological and physiological hypertrophies are regulated by distinct gene programs. Eur J Cardiovasc Prev Rehabil 16:690–697
Samak M, Fatullayev J, Sabashnikov A, Zeriouh M, Schmack B, Farag M, Popov AF, Dohmen PM, Choi YH, Wahlers T, Weymann A (2016) Cardiac hypertrophy: an introduction to molecular and cellular basis. Med Sci Monit Basic Res 22:75–79
Swynghedauw B (1999) Molecular mechnaisms of myocardial remodeling. Physiol Rev 79:215–262
Chien KR, Zhu H, Knowlton KU, Miller-Hance W, van-Bilsen M, O'Brien TX, Evans SM (1993) Transcriptional regulation during cardiac growth and development. Annu Rev Physiol 55:77–95
Taegtmeyer H, Sen S, Vela D (2010) Return to the fetal gene program. Ann N Y Acad Sci 1188:191–198
Mann DL (2014) The evolution of modern theory and therapy for heart failure. Prog Pediatr Cardiol 37:9–12
Mann DL, Bristow MR (2005) Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation 111:2837–2849
Hartupee J, Mann DL (2017) Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 14:30–38
Wachtell K, Smith G, Gerdts E, Dahlöf B, Nieminen MS, Papademetriou V, Bella JN, Ibsen H, Rokkedal J, Devereux RB (2000) Left ventricular filling patterns in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Losartan intervention for endpoint. Am J Cardiol 85:466–472
Verdecchia P, Carini G, Circo A, Dovellini E, Giovannini E, Lombardo M, Solinas P, Gorini M, Maggioni AP, MAVI (MAssa Ventricolare sinistra nell'Ipertensione) Study Group (2001) Left ventricular mass and cardiovascular morbidity in essential hypertension: the MAVI study. J Am Coll Cardiol 38:1829–1835
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH (1991) Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114:345–352
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med 322:1561–1566
Borbely A, Falcao-Pires I, van Heerebeek L et al (2009) Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res 104:780–786
Roe AT, Aronsen JM, Skårdal K et al (2017) Increased passive stiffness promotes diastolic dysfunction despite improved Ca21 handling during left ventricular concentric hypertrophy. Cardiovasc Res 113:1161–1172
Velagalet RS, Gona P, Pencina MJ et al (2014) Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol 113:117–122
Lovic D, Manolis AJ, Lovic B et al (2014) The pathophysiological basis of carotid baroreceptor stimulation for the treatment of resistant hypertension. Curr Vasc Pharmacol 12:16–27
Mann DL (1999) Mechanisms and models in heart failure: a combinatorial approach. Circulation 100:999–1008
Bisping E, Wakula P, Poteser M, Heinzel FR (2014) Targeting cardiac hypertrophy: toward a causal heart failure therapy. J Cardiovasc Pharmacol 64:293–301
Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J Jr, Müller W, Chien KR (1999) Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 97:189–198
Michels da Silva D, Langer H, Graf T (2019) Inflammatory and molecular pathways in heart failure-ischemia. HFpEF and Transthyretin Cardiac Amyloidosis Int J Mol Sci 20(E):2322
Travers JG (2016) The cardiac fibrosis. Circ Res 118:1021–1040
Lourenco AP, Leite-Moreira AF, Balligand JL et al (2018) An integrative translational approach to study heart failure with preserved ecejtion fraction: a position paper from the working group on myocardial function of the European Society of Cardiology. Eur J Heart Fail 20:216–227
Lammerding J, Kamm RD, Lee RT (2004) Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci 1015:53–70
Opie L (2004) Heart physiology. Lippincott, Williams & Wilkins, Philadelphia
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM (2010) Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 3:588–595
Chatterjee NA, Fifer MA (2011) Heart failure. In: Lilly LS (ed) Pathophysiology of heart failure: a collaborative project of medical students and faculty. Lippincott Williams & Wilkins, Philadelphia , chapter 9, pp 216–243
Gaasch WH, Bing OH, Mirsky I (1982) Chamber compliance and myocardial stiffness in left ventricular hypertrophy. Eur Heart J 3 (Suppl A): 139–145
Alderman EL, Glantz SA (1976) Acute hemodynamic interventions shift the diastolic pressure-volume curve in man. Circulation 54:662–671
Gaasch WH, Zile MR (2004) Left ventricular diastolic dysfunction and diastolic heart failure. Annu Rev Med 54:373–394
Brutsaert DL, Sys SU, Gillebert TC (1993) Diastolic failure: pathophysiology and therapeutic implications. J Am Coll Cardiol 22:318–325
Grossman W (2000) Defining diastolic dysfunction. Circulation 101:2020–2021
Vachiery JL, Adir Y, Barberà JA et al (2013) Pulmonary hypertension due to left heart diseases. J am Coll Cardiol 62(Suppl D):D 100–D 108
Little WC (2005) Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation 112:2888–2890
Tokola H, Hautala N, Marttila M, Magga J, Pikkarainen S, Kerkelä R, Vuolteenaho O, Ruskoaho H (2001) Mechanical load-induced alterations in B-type natriuretic peptide gene expression. Can J Physiol Pharmacol 79:646–653
Vanderheyden M, Bartunek J, Goethals M (2004) Brain and other natriuretic peptides: molecular aspects. Eur J Heart Fail 6:261–268
Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL, LeWinter M (2015) Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131:1247–1259
Adir Y, Guazzi M, Offer A, Temporelli PL, Cannito A, Ghio S (2017) Pulmonary hemodynamics in heart failure with reduced or preserved ejection fraction and pulmonary hypertension: similarities and disparities. Am Heart J 192:120–127
Brucks S, Little WC, Chao T, Kitzman DW, Wesley-Farrington D, Gandhi S, Shihabi ZK (2005) Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol 95:603–606
Iwano H, Little WC (2013) Heart failure: what does ejection fraction have to do with it? J Cardiol 62:1–3
Galie N, Humbert M, Vachiery JL et al (2016) The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Eur Heart J 37:67–119
Haddad F, Doyle R, Murphy DJ, Hunt SA (2008) Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117:1717–1731
Borlaug BA, Reddy YNV (2019) The role of the pericardium in heart failure. JACC: Heart Fail 7:574–585
Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C, American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia (2018) Evaluation and Management of Right-Sided Heart Failure. Circulation 137:e578–e622
Voelkel NF, Natarajan R, Drake JI, Boogard HJ (2011) Right ventricle in pulmonary hypertension. Comp Physiol 1:525–540
Vonk Noordegraaf A, Chin KM, Haddad F et al (2019) Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 53: pii: 1801900
Samson N, Pauli R (2017) Epigenetics, inflammation and metabolism in right heart failure associated with pulmonary hypertension. Pulmonary Circulation 7:572–587
Borgdorff MAJ, Dickinson MG, Berger RMF, Bartelds B (2015) Right ventricular failure due to chronic pressure load: what have we learned in animal models since the NIH working group statement? Heart Fail Rev 20:475–491
Naeije R, Brimioulle S, Dewachter L (2014) Biomechanics of the right ventricle in health and disease (2013 Grover conference series). Pulmonary Circ 4:395–406
Borgdorff MA, Bartelds B, Dickinson MG et al (2013) Distinct loading conditions reveal various patterns of right ventricular adaptation. Am J Physiol heart Circ Physiol 305:H 354–H 364
Cingolani HE, Pérez NG, Cingolani OH, Ennis IL (2013) The Anrep effect: 100 years later. Am J Physiol heart Circ Physiol 304:H 175–H 182
Szabó G, Soós P, Bährle S, Radovits T, Weigang E, Kékesi V, Merkely B, Hagl S (2006) Adaptation of the right ventricle to an increased afterload in the chronically volume overloaded heart. Ann Thorac Surg 82:989–995
Danton MH, Greil GF, Byrne JG et al (2003) Right ventricular volume measurement by conductance catheter. Am J Physiol Heart Circ Physiol 285:H 1774–H 1785
Belenkie I, Smith ER, Tyberg JV (2001) Ventricular interaction: from bench to bedside. Ann Med 33:236–241
Jardin F, Dubourg O, Guéret P, Delorme G, Bourdarias J-P (2001) Quantitative two-dimensional echocardiography in massive pulmonary embolism: emphasis on ventricular interdependence and leftward septal displacement. J Am Coll Cardiol 10:1201–1206
Rain S, Handoko ML, Trip P et al (2013) Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 128:2016–2025
de Man FS, Handoko ML, van Ballegoij JJ, Schalij I, Bogaards SJ, Postmus PE, van der Velden J, Westerhof N, Paulus WJ, Vonk-Noordegraaf A (2012) Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ: Heart Fail 5:97–105
Bartelds B, Borgdorff MA, Smit-van Oosten A, Takens J, Boersma B, Nederhoff MG, Elzenga NJ, van Gilst W, de Windt LJ, Berger RM (2011) Differential responses of the right ventricle to abnormal loading conditions in mice: pressure vs. volume load. Eur J Heart Fail 13:1275–1282
Borgdorff MAJ, Bartelds B, Dickinson MG et al (2012) Sildenafil enhances systolic adaptation, but does not prevent diastolic dysfunction, in the pressure-loaded right ventricle. Eur J Heart Fail 14:1067–1074
Bossers GPL, Hagdorn QAJ, Ploegstra MJ et al (2018) Volume load-induced right ventricular dysfunction in animal models: insights in a translational gap in congenital heart disease. Eur J Heart Fail 20:80–812
Reddy S, Zhao M, Hu DQ et al (2013) Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol heart Circ Physiol 304:H 1313–H 1327
Davlouros PA, Niwa K, Webb GD, Gatzoulis MA (2006) The right ventricle in congenital heart disease. Heart 92 (Suppl I): i27–i38
Messika-Zeitoun D, Thomson H, Bellamy M, Scott C, Tribouilloy C, Dearani J, Tajik AJ, Schaff H, Enriquez-Sarano M (2004) Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg 128:296–302
Apitz C, Webb GD, Redington AN (2009) Tetralogy of Fallot. Lancet 374:1462–1471
Reddy S, Bernstein D (2008) Molecular aspects of RV adaptation to stress. In: Friedberg MK, Redington AN (eds) RV physiology, adaptation and failure in congenital andaquired heart disease. Springer Nature, Cham, Switzerland, chapter 3, p 35
Melaku LS, Desalegn T (2019) Molecular mediators, characterization of signaling pathways with description of cellular distinctions in pathophysiology of cardiac hypertrophy and molecular changes underlying a transition to heart failure. Int J Health Allied Sci 8:1–24
Sadoshima J, Izumo S (1993) Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J 12:1681–1692
Franssen C, Chen S, Unger A et al (2016) Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. J Am Coll Cardiol: Heart Fail 4:312–324
Hamdani N, Franssen C, Lourenço A et al (2013) Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail 6:1239–1249
Lyon RC, Zanella F, Omens JH, Sheikh F (2015) Mechanotransduction in cardiac hypertrophy and failure. Circ Res 116:1462–1476
Sadoshima J, Izumo S (1997) The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 59:551–571
Pikkarainen S, Tokola H, Ruskoaho H (2007) Mechanotransduction of the endocrine heart: paracrine and intracellular regulation of B-type natriuretic peptide synthesis. In: Weckström M, Tavi P (eds) Cardiac Mechanotransduction, Landes bioscience and springer science and business, New York, NY, chapter, vol 9, pp 134–144
Liang F, Gardner DG (1999) Mechanical strain activates BNP gene transcription through a p38/NF-κB–dependent mechanism. J Clin Invest 104:1603–1612
De Keulenaer GW, Brutsaert DL (2011) Systolic and diastolic heart ailure are overlapping phenotypes within the heart failure spectrum. Circulation 123:1996–2004
Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ (2016) Phenotype- specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134:73–90
Gondalia RB, Rothermel BA, Lavandero S, Gillette TG, Hill JA (2012) Cardiac plasticity in health and disease. In: Patterson C, Willis MS (eds) Translational cardiology. Humana Press, Totowa, NJ, Springer Science and Business Media, pp 185–250
Mannacio V, Antignano A, De Amicis V et al (2013) B-type natriuretic peptide as a biochemical marker of left ventricular diastolic function: assessment in asymptomatic patients 1 year after valve replacement for aortic stenosis. Interact Cardiovasc Thorac Surg 17:371–377
Watanabe S, Shite J, Takaoka H, Shinke T, Imuro Y, Ozawa T, Otake H, Matsumoto D, Ogasawara D, Paredes OL, Yokoyama M (2006) Myocardial stiffness is an important determinant of the plasma brain natriuretic peptide concentration in patients with both diastolic and systolic heart failure. Eur Heart J 27:832–838
Yang F, Dong A, Mueller P et al (2012) Coronary artery remodeling in a model of left ventricular pressure overload is influenced by platelets and inflammatory cells. PLoS One 7:e40196
Smeets PJH, Teunissen BE, Planavila A et al (2008) Inflammatory pathways are activated during Cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors PPARα and PPARδ. J Biol Chem 283:29109–21118
Lam CSP, Lund LH (2016) Microvascular endothelial dysfunction in heart failure with preserved ejection fraction. Heart 102:255–256
Ferrari R (2016) Heart failure: an historical perspective, Europ Heart J Supplements. 18(Suppl G):G 3–G 10
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762
Floras JS (2009) Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol 54:375–385
Van Heerebeek L, Paulus WJ (2016) Understanding heart failure with preserved ejection fraction: where are we today? Neth Heart J 24:227–236
Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie R, Komajda M, McMurray J, Lindenfeld J (2015) Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 17:925–935
Khan MS, Fonarow GC, Khan H, Greene SJ, Anker SD, Gheorghiade M, Butler J (2017) Renin-angiotensin blockade in heart failure with preserved ejection fraction: a systematic review and meta-analysis. ESC Heart Fail 4:402–408
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krueger, W., Bender, N., Haeusler, M. et al. The role of mechanotransduction in heart failure pathobiology—a concise review. Heart Fail Rev 26, 981–995 (2021). https://doi.org/10.1007/s10741-020-09915-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09915-1