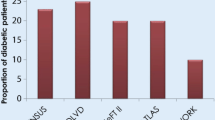
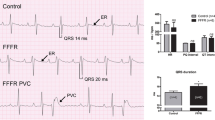
Abstract
Diabetes mellitus (DM) is a major and worsening global health problem, currently affecting over 450 million people and reducing their quality of life. Type 2 diabetes mellitus (T2DM) accounts for more than 90% of DM and the global epidemic of obesity, which largely explains the dramatic increase in the incidence and prevalence of T2DM in the past 20 years. Obesity is a major risk factor for DM which is a major cause of morbidity and mortality in diabetic patients. The electro-mechanical function of the heart is frequently compromised in diabetic patients. The aim of this review is to discuss the pathophysiology of electro-mechanical dysfunction in the diabetic heart and in particular, the Zucker diabetic fatty (ZDF) rat heart, a well-studied model of T2DM and obesity.






Similar content being viewed by others
References
International Diabetes Federation (2017) IDF Diabetes Atlas, 8th edn. Int. Diabetes Fed, pp 40–95
Mayfield J (1998) Diagnosis and Classification of Diabetes Mellitus: New Criteria. Am Fam Physician 58:1355–1362. https://doi.org/10.2337/dc14-S081
Low Wang CC, Hess CN, Hiatt WR, Goldfine AB (2016) Clinical update: Cardiovascular disease in diabetes mellitus. Circulation 133:2459–2502. https://doi.org/10.1161/CIRCULATIONAHA.116.022194
Altunkeser BB, Ceylan E, Demir K, Yilmaz A, Avci A, Kaya Z, Ersecgin A, Marakoglu K, Armutlukuyu M (2015) Assessment of atrial electromechanical delay and P-wave dispersion in patients with type 2 diabetes mellitus. J Cardiol 67:378–383. https://doi.org/10.1016/j.jjcc.2015.06.003
World Health Organization (2018) Fact Sheets. Obesity and overweight. In: WHO. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight
NHLBI Obesity Education Initiative Expert Panel. National Institutes of Health. National Heart Lung and Blood Institute. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. NIH Publ No. 00-4084
Swinburn BA, Kraak VI, Allender S et al (2019) The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 393:791–846. https://doi.org/10.1016/S0140-6736(18)32822-8
Gurevich-Panigrahi T, Panigrahi S, Wiechec E, Los M (2009) Obesity: pathophysiology and clinical management. Curr Med Chem 16:506–521
Dobbs R, Swinburn B (2015) The global obesity threat. In: Projuct Synd. McKinsey Glob. Inst. https://www.mckinsey.com/mgi/overview/in-the-news/the-global-obesity-threat. Accessed 6 Jun 2019
Vasanji Z, Dhalla NS, Netticadan T (2004) Increased inhibition of SERCA2 by phospholamban in the type I diabetic heart. Mol Cell Biochem 261:245–249
Howarth FC, Parekh K, Jayaprakash P, Inbaraj ES, Oz M, Dobrzynski H, Adrian TE (2017) Altered profile of mRNA expression in atrioventricular node of streptozotocin-induced diabetic rats. Mol Med Rep 16:3720–3730. https://doi.org/10.3892/mmr.2017.7038
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA (2001) Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J Clin Endocrinol Metab 86:1930–1935. https://doi.org/10.1210/jcem.86.5.7463
Yokoi N, Hoshino M, Hidaka S, Yoshida E, Beppu M, Hoshikawa R, Sudo K, Kawada A, Takagi S, Seino S (2013) A Novel Rat Model of Type 2 Diabetes: The Zucker Fatty Diabetes Mellitus ZFDM Rat. J Diabetes Res 2013:103731–103740. https://doi.org/10.1155/2013/103731
Harvey RA, Ferrier D (2011) In: Harvey RA, Ferrier D (eds) Lippincott’s Illustrated Reviews: Biochemistry, 5th edn. Wolters Kluwer Health, Lippincott Williams & Wilkins, Philadelphia, pp 307–356
Klabunde RE (2017) Cardiac electrophysiology: normal and ischemic ionic currents and the ECG. Adv Physiol Educ 41:29–37. https://doi.org/10.1152/advan.00105.2016
Crawford M, DiMarco J, Paulus W (2009) Cardiology. https://www.elsevier.com/books/cardiology/crawford/978-0-7234-3485-6. Accessed 10 Oct 2018
Boyett MR (2009) “And the beat goes on” the cardiac conduction system: The wiring system of the heart. Exp Physiol 94:1035–1049. https://doi.org/10.1113/expphysiol.2009.046920
Kashou AH, Kashou HE (2018) Physiology, Sinoatrial Node (SA Node). In: StatPearls. http://www.ncbi.nlm.nih.gov/pubmed/29083608. Accessed 10 Nov 2018
Bartos DC, Grandi E, Ripplinger CM (2015) Ion channels in the heart. Compr Physiol 5:1423–1464. https://doi.org/10.1002/cphy.c140069
Pinnell J, Turner S, Howell S (2007) Cardiac muscle physiology. Contin Educ Anaesth Crit Care Pain 7:85–88. https://doi.org/10.1093/bjaceaccp/mkm013
Hafeez Y, Grossman SA (2018) Rhythm, Junctional. In: StatPearls [internet]. http://www.ncbi.nlm.nih.gov/pubmed/29939537. Accessed 10 Nov 2018
Czick ME, Shapter CL, Silverman DI (2016) Atrial Fibrillation: The Science behind Its Defiance. Aging Dis 7:635–656. https://doi.org/10.14336/AD.2016.0211
Silverthorn DU, Ober WC, Garrison CW, Silverthorn AC, Johnson BR (2007) Human physiology : an integrated approach. In: Regulation, 5th edn. Pearson Education Inc/Benjamin Cummings, San Francisco, pp 467–545
Pfeiffer ER, Tangney JR, Omens JH, McCulloch AD (2014) Biomechanics of cardiac electromechanical coupling and mechanoelectric feedback. J Biomech Eng 136:021007–021018. https://doi.org/10.1115/1.4026221
Bers DM, Despa S (2013) Cardiac Excitation-Contraction Coupling. Encycl Biol Chem Second Ed 415:379–383. https://doi.org/10.1016/B978-0-12-378630-2.00221-8
Landstrom AP, Dobrev D, Wehrens XHT (2017) Calcium Signaling and Cardiac Arrhythmias. Circ Res 120:1969–1993
Shih H-TT (1994) Anatomy of the Action Potential in the Heart. Tex Heart Inst J 21:30–41
Towbin JA (2004) Molecular genetic basis of sudden cardiac death. Pediatr Clin N Am 51:1229–1255. https://doi.org/10.1016/j.pcl.2004.04.012
Snyders DJ (1999) Structure and function of cardiac potassium channels. Cardiovasc Res 42:377–390. https://doi.org/10.1016/S0008-6363(99)00071-1
Hille B (1978) Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys J 22:283–294. https://doi.org/10.1016/S0006-3495(78)85489-7
Huang H, Pugsley MK, Fermini B, Curtis MJ, Koerner J, Accardi M, Authier S (2017) Cardiac voltage-gated ion channels in safety pharmacology: Review of the landscape leading to the CiPA initiative. J Pharmacol Toxicol Methods 87:11–23. https://doi.org/10.1016/j.vascn.2017.04.002
Schaffer SW (1991) Cardiomyopathy associated with noninsulin-dependent diabetes. Mol Cell Biochem 107:1–20. https://doi.org/10.1007/BF02424571
Mortuza R, Chakrabarti S (2014) Glucose-induced cell signaling in the pathogenesis of diabetic cardiomyopathy. Heart Fail Rev 19:75–86. https://doi.org/10.1007/s10741-013-9381-z
Falcão-Pires I, Leite-Moreira AF (2012) Diabetic cardiomyopathy: Understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail Rev 17:325–344. https://doi.org/10.1007/s10741-011-9257-z
Isfort M, Stevens SCW, Schaffer S, Jong JC, Wold LE (2014) Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail Rev 19:35–48. https://doi.org/10.1007/s10741-013-9377-8
Murarka S, Movahed MR (2010) Diabetic Cardiomyopathy. J Card Fail 16:971–979. https://doi.org/10.1016/J.CARDFAIL.2010.07.249
Matafome P, Rodrigues T, Sena C, Seiça R (2017) Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med Res Rev 37:368–403. https://doi.org/10.1002/med.21410
Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, Mori K, Tamura N, Hosoda K, Nakao K (1996) Molecular cloning of rat leptin receptor isoform complementary DNAs-identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun 225:75–83. https://doi.org/10.1006/bbrc.1996.1133
Bray GA (1977) The Zucker-fatty rat: a review. Fed Proc 36:148–153
Baynes JW, Murray DB (2009) The Metal Chelators, Trientine and Citrate, Inhibit the Development of Cardiac Pathology in the Zucker Diabetic Rat. Exp Diabetes Res 2009:696378–696384. https://doi.org/10.1155/2009/696378
Raza H, John A, Howarth FC (2012) Alterations in Glutathione Redox Metabolism, Oxidative Stress, and Mitochondrial Function in the Left Ventricle of Elderly Zucker Diabetic Fatty Rat Heart. Int J Mol Sci 13:16241–16254. https://doi.org/10.3390/ijms131216241
Howarth FC, Qureshi MA, Hassan Z, Al Kury LT, Isaev D, Parekh K, Yammahi SRKDKD, Oz M, Adrian TE, Adeghate E (2011) Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+signalling in young type 2 Zucker diabetic fatty rat heart. Exp Physiol 96:325–337. https://doi.org/10.1113/expphysiol.2010.055574
Howarth FC (2012) Ventricular myocyte contraction, intracellular calcium and expression of genes encoding cardiac muscle proteins in young and aging Zucker diabetic fatty rat heart reviewed. Hamdan Med J 5:165–172. https://doi.org/10.7707/hmj.v5i2.140
Sista AK, O’Connell MK, Hinohara T, Oommen SS, Fenster BE, Glassford AJ, Schwartz EA, Taylor CA, Reaven GM, Tsao PS (2005) Increased aortic stiffness in the insulin-resistant Zucker fa/fa rat. Am J Physiol Heart Circ Physiol 289:H845–H851. https://doi.org/10.1152/ajpheart.00134.2005
Lum-Naihe K, Toedebusch R, Mahmood A, Bajwa J, Carmack T, Kumar SA, Ardhanari S, Demarco VG, Emter CA, Pulakat L (2017) Cardiovascular disease progression in female Zucker Diabetic Fatty rats occurs via unique mechanisms compared to males. Sci Rep 7:17823–17839. https://doi.org/10.1038/s41598-017-18003-8
Kim GH (2013) MicroRNA regulation of cardiac conduction and arrhythmias. Transl Res 161:381–392. https://doi.org/10.1016/j.trsl.2012.12.004
Delic D, Eisele C, Schmid R, Luippold G, Mayoux E, Grempler R (2016) Characterization of micro-RNA changes during the progression of type 2 diabetes in Zucker diabetic fatty rats. Int J Mol Sci 17:665–681. https://doi.org/10.3390/ijms17050665
Fernandes T, Casaes L, Soci Ú, Silveira A, Gomes J, Barretti D, Roque F, Oliveira E (2018) Exercise Training Restores the Cardiac Microrna-16 Levels Preventing Microvascular Rarefaction in Obese Zucker Rats. Obes Facts 11:15–24. https://doi.org/10.1159/000454835
Bruinstroop E, Eliveld J, Foppen E, Busker S, Ackermans MT, Fliers E, Kalsbeek A (2015) Hepatic denervation and dyslipidemia in obese Zucker (fa/fa) rats. Int J Obes 39:1655–1658. https://doi.org/10.1038/ijo.2015.122
Radovits T, Korkmaz S, Mátyás C, Oláh A, Németh BT, Páli S, Hirschberg K, Zubarevich A, Gwanmesia PN, Li S, Loganathan S, Barnucz E, Merkely B, Szabó G (2015) An Altered Pattern of Myocardial Histopathological and Molecular Changes Underlies the Different Characteristics of Type-1 and Type-2 Diabetic Cardiac Dysfunction. J Diabetes Res 2015:728741–728753. https://doi.org/10.1155/2015/728741
Mourmoura E, Vial G, Laillet B, Rigaudière J-P, Hininger-Favier I, Dubouchaud H, Morio B, Demaison L (2013) Preserved endothelium-dependent dilatation of the coronary microvasculature at the early phase of diabetes mellitus despite the increased oxidative stress and depressed cardiac mechanical function ex vivo. Cardiovasc Diabetol 12:49–66. https://doi.org/10.1186/1475-2840-12-49
Laughlin MH, Woodman CR, Ray CA, Behnke BJ, Lesniewski LA, Donato AJ, Delp MD (2008) Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Circ Physiol 294:H1840–H1850. https://doi.org/10.1152/ajpheart.00692.2007
Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC (2006) Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. AJP Cell Physiol 292:C1370–C1378. https://doi.org/10.1152/ajpcell.00422.2006
Daniels LJ, Wallace RS, Nicholson OM, Wilson GA, McDonald FJ, Jones PP, Baldi JC, Lamberts RR, Erickson JR (2018) Inhibition of calcium/calmodulin-dependent kinase II restores contraction and relaxation in isolated cardiac muscle from type 2 diabetic rats. Cardiovasc Diabetol 17:89–104. https://doi.org/10.1186/s12933-018-0732-x
Burgdorf C, Richardt G, Schütte F, Dendorfer A, Kurz T (2006) Impairment of presynaptic α2-adrenoceptor-regulated norepinephrine overflow in failing hearts from Zucker diabetic fatty rats. J Cardiovasc Pharmacol 47:256–262. https://doi.org/10.1097/01.fjc.0000202560.61667.3e
Bonde L, Shokouh P, Jeppesen PB, Boedtkjer E (2017) Crosstalk between cardiomyocyte-rich perivascular tissue and coronary arteries is reduced in the Zucker Diabetic Fatty rat model of type 2 diabetes mellitus. Acta Physiol 219:227–238. https://doi.org/10.1111/apha.12685
Wang P, Zeng H, Lloyd SG, Bonen A, Chatham JC (2004) Impact of altered substrate utilization on cardiac function in isolated hearts from Zucker diabetic fatty rats. Am J Physiol Circ Physiol 288:H2102–H2110. https://doi.org/10.1152/ajpheart.00935.2004
Holloway GP, Snook LA, Harris RJ, Glatz JFCC, Luiken JJFPFP, Bonen A (2011) In obese Zucker rats, lipids accumulate in the heart despite normal mitochondrial content, morphology and long-chain fatty acid oxidation. J Physiol 589:169–180. https://doi.org/10.1113/jphysiol.2010.198663
VanHoose L, Sawers Y, Loganathan R, Vacek JL, Stehno-Bittel L, Novikova L, Al-Jarrah M, Smirnova IV (2010) Electrocardiographic changes with the onset of diabetes and the impact of aerobic exercise training in the Zucker Diabetic Fatty (ZDF) rat. Cardiovasc Diabetol 9:56–66. https://doi.org/10.1186/1475-2840-9-56
Lin Y-CC, Hull R, Huang J, Martin KH, Davis M, Hileman S, Yu H-GG, Martin KH, Hull R, Davis M, Yu H-GG (2015) Leptin decreases heart rate associated with increased ventricular repolarization via its receptor. Am J Physiol Circ Physiol 309:H1731–H1739. https://doi.org/10.1152/ajpheart.00623.2015
Wilson C, Lincoln C (1984) Beta-adrenoceptor subtypes in human, rat, guinea pig, and rabbit atria. J Cardiovasc Pharmacol 6:1216–1221
Hardie DG, Hawley SA, Scott JW (2006) AMP-activated protein kinase - development of the energy sensor concept. J Physiol 574:7–15. https://doi.org/10.1113/jphysiol.2006.108944
Li J, Yan B, Huo Z, Liu Y, Xu J, Sun Y, Liu Y, Liang D, Peng L, Zhang Y, Zhou Z-N, Shi J, Cui J, Chen Y-H (2010) β 2 - but not β 1 -adrenoceptor activation modulates intracellular oxygen availability. J Physiol 588:2987–2998. https://doi.org/10.1113/jphysiol.2010.190900
Pérez-Schindler J, Philp A, Baar K, Hernández-Cascales J (2011) Regulation of contractility and metabolic signaling by the β2-adrenergic receptor in rat ventricular muscle. Life Sci 88:892–897. https://doi.org/10.1016/j.lfs.2011.03.020
Bussey CT, Thaung HPA, Hughes G, Bahn A, Lamberts RR (2018) Cardiac β-adrenergic responsiveness of obese Zucker rats: The role of AMPK. Exp Physiol 103:1067–1075. https://doi.org/10.1113/EP087054
Cook RF, Bussey CT, Fomison-Nurse IC, Hughes G, Bahn A, Cragg PA, Lamberts RR (2019) β2 -Adrenoceptors indirectly support impaired β1 -adrenoceptor responsiveness in the isolated type 2 diabetic rat heart. Exp Physiol 104:808–818. https://doi.org/10.1113/EP087437
Jensen RV, Jespersen NR, Støttrup NB, Povlsen JA, Hjortbak MV, Bøtker HE, Laursen MR, Hjort J, Løfgren B (2018) Influence of diabetes mellitus duration on the efficacy of ischemic preconditioning in a Zucker diabetic fatty rat model. PLoS One 13:e0192981–e0192997. https://doi.org/10.1371/journal.pone.0192981
Onal B, Gratz D, Hund TJ (2017) Ca2+/calmodulin-dependent kinase II-dependent regulation of atrial myocyte late Na+ current, Ca2+ cycling, and excitability: a mathematical modeling study. Am J Physiol Heart Circ Physiol 313:H1227–H1239. https://doi.org/10.1152/ajpheart.00185.2017
Schach C, Resch M, Schmid PM, Riegger GA, Endemann DH (2014) Type 2 diabetes: increased expression and contribution of IK Ca channels to vasodilation in small mesenteric arteries of ZDF rats. Am J Physiol Circ Physiol 307:H1093–H1102. https://doi.org/10.1152/ajpheart.00240.2013
Kléber AG, Rudy Y (2004) Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84:431–488. https://doi.org/10.1152/physrev.00025.2003
Ploug T, Braunstein TH, Olsen KB, Holstein-Rathlou N-H, Axelsen LN, Sørensen CM, Nielsen MS, Andersen CB (2013) Myocardial impulse propagation is impaired in right ventricular tissue of Zucker Diabetic Fatty (ZDF) rats. Cardiovasc Diabetol 12:19–30. https://doi.org/10.1186/1475-2840-12-19
Benke K, Braun S, Mátyás C, Barta BA, Merkely B, Ruppert M, Lakatos BK, Kovács A, Németh BT, Oláh A, Radovits T, Tokodi M (2018) Comparison of speckle-tracking echocardiography with invasive hemodynamics for the detection of characteristic cardiac dysfunction in type-1 and type-2 diabetic rat models. Cardiovasc Diabetol 17:13–26. https://doi.org/10.1186/s12933-017-0645-0
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sultan, A., Singh, J. & Howarth, F.C. Mechanisms underlying electro-mechanical dysfunction in the Zucker diabetic fatty rat heart: a model of obesity and type 2 diabetes. Heart Fail Rev 25, 873–886 (2020). https://doi.org/10.1007/s10741-019-09872-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09872-4