Abstract
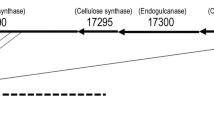
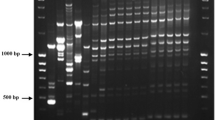
A sensitive and specific assay was developed to detect bacterial leaf and flower spot disease on zinnia caused by Xanthomonas campestris pv. zinniae (Xcz). A draft genome sequence was determined for Xcz strain E4, isolated from zinnia in Jilin province, China. Comparative genomics analyses were utilized to identify a sequence specific to Xcz in the unique gene 0834. This sequence region was amplified as 1044 bp DNA fragment using designed primers 0834F and 0834R. Using this primer set, specific PCR products were only amplified from DNA from Xcz strains and not from other pathovars of X. campestris, other species of Xanthomonas, or from other genera such as Pseudomonas and Bacillus. The limit of PCR detection in pure culture suspension was approximately 1.7 × 103 CFU/ml per reaction. Specific amplification of the fragments for Xcz was sensitive relatively to the technique used, detecting as low as 0.1 pg template DNA. The PCR technique was also applied to detect the Xcz pathogen in naturally infected leaves and seeds of zinnia.





Similar content being viewed by others
References
Alippi, A. M. (1985). Bacterial leaf spot of Zinnia elegans, a new disease in Argentina. Rev Argent Microbiol, 17, 11–13.
Araújo, E. R., Costa, J. R., Ferreira, M. A. S. V., & Quezado-Duval, A. M. (2012). Simultaneous detection and identification of the Xanthomonas species complex associated with tomato bacterial spot using species-specific primers and multiplex. J Appl Microbiol, 113, 1479–1490.
An, J. H., Noh, Y. H., & Kim, Y. E. (2015). Development of PCR and TaqMan PCR assays to detect Pseudomonas coronafaciens, a causal agent of halo blight of oats. Plant Pathol J, 31, 25–32.
Akhtar, M. A., & Khokhar, L. K. (1988). Bacterial leaf spot of zinnia cultivars caused by Xanthomonas campestris pv. zinniae. Pakistan J. Agric Res, 9, 209–211.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. J Mol Biol, 215, 403–410.
Bühlmann, A., Pothier, J. F., Tomlinson, J. A., Frey, J. E., Boonham, N., Smits, T. H. M., et al. (2013). Genomics- informed design of loop-mediated isothermal amplification for detection of phytopathogenic Xanthomonas arboricola pv. pruni at the intraspecific level. Plant Pathol, 62, 475–484.
Bertus, A. L., & Hayward, A. C. (1971). A bacterial leaf spot of zinnia in New South Wales. Proc Linnean Soc NSW, 96, 81–84.
Baek, K. Y., Lee, H. H., Son, G. J., Lee, P. A., Roy, N., Seo, Y. S., & Lee, S. W. (2018). Specific and sensitive primers developed by comparative genomics to detect bacterial pathogens in grains. Plant Pathol J, 34, 104–112.
Bahar, O., Efrat, M., Hadar, E., Dutta, B., Walcott, R. R., & Burdman, S. (2008). New subspecies- specific polymerase chain reaction-based assay for the detection of Acidovorax avenae subsp. citrulli. Plant Pathol, 57, 754–763.
Chiaromonte, F., Yap, V. B., & Miller, W. (2001). Scoring pairwise genomic sequence alignments. Pac Symp Biocomput, 7, 115.
Deighton, F. C. (1957). Plant pathology section. Review of Applied Mycology, 36, 381.
Harris, R. S. (2007). Improved pairwise alignment of genomic DNA. ProQuest.
Holcomb, G. E. (1985). First report of bacterial leaf and flower spot of zinnia in Louisana. Plant Dis, 69, 360.
Hernándezy, Y., & Trujillo, G. (2000). Xanthomonas campestris pv. zinniae, infecting Zinnia plants (Zinnia elegans Jacq.) in Venezuela. Rev Fac Agron (LUZ), 17, 156–163.
Kurtz, S., Phillippy, A., Delcher, A. L., Smoot, M., Shumway, M., Antonescu, C., et al. (2004). Versatile and open software for comparing large genomes. Genome Biol, 5, R12.
Li, W., & Godzik, A. (2006). Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics, 22, 1658–1659.
Li, W., Jaroszewski, L., & Godzik, A. (2001). Clustering of highly homologous sequences to reduce the size of large protein database. Bioinformatics, 17, 282–283.
Li, W., Jaroszewski, L., & Godzik, A. (2002). Tolerating some redundancy significantly speeds up clustering of large protein databases. Bioinformatics, 18, 77–82.
Lang, J. M., Hamilton, J. P., Diaz, M. G. Q., Van Sluys, M. A., Burgos, M. R. G., Vera Cruz, C. M., et al. (2010). Genomics-based diagnostic marker development for Xanthomonas oryzae pv. oryzae and X. oryzae pv. oryzicola. Plant Dis, 94, 311–319.
Larrea-Sarmiento, A., Dhakal, U., Boluk, G., Fatdal, L., Alvarez, A., Strayer-Scherer, A., et al. (2018). Development of a genome-informed loop-mediated isothermal amplification assay for rapid and specific detection of Xanthomonas euvesicatoria. Sci Rep, 8, 14298–14309.
Myung, I. S., Lee, J. Y., Yoo, H. L., Wu, J. M., & Shim, H. S. (2012). Bacterial leaf spot of zinnia caused by Xanthomonas campestris pv. zinniae, a new disease in Korea. Plant Dis, 96, 1064.
Minoru, T. (1982). Xanthomonas campestris pv. zinniae (Hopkins & Dowson 1949) dye 1978. Ann. Phytopath. Soc. Japan, 48, 532–533.
Nanizzi, A. (1929). Una bacteriosi della ‘Zinnia elegans’ Jaqc. (Notapreliminare). Atti Report. Accademia Fisiocritici Siena. Ser. X, 416-417.
Peregrine, W. T., & Siddiqi, M. A. (1972). A revised and annotated list of plant disease in Malawi.
Pritchard, L., Humphris, S., Saddler, G. S., Parkinson, N. M., Bertrand, V., Elphinstone, J. G., et al. (2013). Detection of phytopathogens of the genus Dickeya using a PCR primer prediction pipeline for draft bacterial genome sequences. Plant Pathol, 62, 587–596.
Robbs, C. F. (1954). Bacterias fitopatogenicas do Brasil. Rio de Journal, 13, 265–282.
Rangaswami, G., & Gowda, S. S. (1963). On some bacterial disease of ornamentals and vegetables in Madras state. Indian Phytopathology, 16, 74–85.
Strider, D. L. (1979). Detection of Xanthomonas nigromaculans f. sp. zinniae in zinnia seed, Plant Dis. Reptr., 63, 869–873.
Sundin, G. W., Wang, N., Charkowski, A. O., Castiblanco, L. F., Jia, H., & Zhao, Y. (2016). Perspectives on the transition from bacterial phytopathogen genomics studies to applications enhancing disease management: From promise to practice. Phytopathology, 106, 1071–1082.
Schwarczinger, I., Vajna, L., & Süle, S. (2008). First report of bacterial leaf and flower spot of Zinnia elegans caused by Xanthomonas campestris pv. zinniae in Hungary. Plant Pathol, 57, 367.
Sleesman, J., White, D. G., & Ellett, C. W. (1973). Bacterial leaf spot of zinnia: A new disease in North America. Plant Dis Rep, 57, 555–557.
Tian, Q., Feng, J. J., Hu, J., & Zhao, W. J. (2016). Selective detection of viable seed-borne Acidovorax citrulli by real-time PCR with propidium monoazide. Sci Rep, 6, 35457.
Walcott, R. R., & Gitaitis, R. D. (2000). Detection of Acidovorax avenae subsp. citrulli in watermelon seed using immunomagnetic separation and the polymerase chain reaction. Plant Dis, 84, 470–474.
Young, J. M., Park, D. C., Shearman, H. M., & Fargier, E. (2008). A multilocus sequence analysis of the genus Xanthomonas. Syst Appl Microbiol, 31, 366–377.
Zhao, Y. T., Lu, B. H., Bai, Q. R., & Gao, J. (2016). First report of Xanthomonas campestris pv. zinniae causing bacterial leaf and flower spot disease of Zinnia elegans in Jilin Province, China. Plant Dis, 100, 208.
Acknowledgments
This study was supported by 111 Project (D17014). We thank Yawen He (College of Life Science and Biotechnology, Shanghai Jiao Tong University), Tingchang Zhao (Institute of Plant Protection, Chinese Academy of Agricultural Sciences), and Xiaoling Deng (College of agriculture, South China Agricultural University) for strains provided by them.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Yt., Sundin, G.W., Zhang, Xy. et al. Sensitive and specific detection of Xanthomonas campestris pv. zinniae by PCR using pathovar-specific primers. Eur J Plant Pathol 156, 491–500 (2020). https://doi.org/10.1007/s10658-019-01898-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01898-6