Abstract
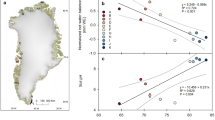
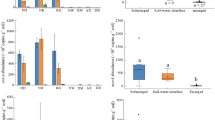
Methane, which is produced by methanogenic archaea, is the second most abundant carbon compound in the atmosphere. Due to its strong radiative forcing, many studies have been conducted to determine its sources, budget, and dynamics. However, a mechanistic model of methane flux has not been developed thus far. In this study, we attempt to examine the relevance of the abundance of methanogen as a biological indicator of methane flux in three different types of soil ecosystems: permafrost, rice paddy, and mountainous wetland. We measured the annual average methane flux and abundance of methanogen in the soil ecosystems in situ. The correlation between methane flux and the abundance of methanogen exists only under a specific biogeochemical conditions such as SOM of higher than 60 %, pH of 5.6–6.4, and water-saturated. Except for these conditions, significant correlations were absent. Therefore, microbial abundance information can be applied to a methane flux model selectively depending on the biogeochemical properties of the soil ecosystem.





Similar content being viewed by others
References
Allison, S. D., Wallenstein, M. D., & Bradford, M. A. (2010). Soil-carbon response to warming dependent on microbial physiology. Nature Geoscience, 3(5), 336–340.
Barnes, R. T., Smith, R. L., & Aiken, G. R. (2012). Linkages between denitrification and dissolved organic matter quality, Boulder Creek watershed, Colorado. Journal of Geophysical Research: Biogeosciences,. doi:10.1029/2011JG001749.
Berger, S., Jang, I., Seo, J., Kang, H., & Gebauer, G. (2013). A record of N2O and CH4 emissions and underlying soil processes of Korean rice paddies as affected by different water management practices. Biogeochemistry, 115(1–3), 317–332.
Cao, M., Marshall, S., & Gregson, K. (1996). Global carbon exchange and methane emissions from natural wetlands: Application of a process-based model. Journal of Geophysical Research: Atmospheres, 101(D9), 14399–14414.
Carney, K. M., & Matson, P. A. (2005). Plant communities, soil microorganisms, and soil carbon cycling: Does altering the world belowground matter to ecosystem functioning? Ecosystems, 8(8), 928–940.
Chen, Y. H., & Prinn, R. G. (2005). Atmospheric modeling of high- and low-frequency methane observations: Importance of interannually varying transport. Journal of Geophysical Research: Atmospheres,. doi:10.1029/2004JD005542.
Conrad, R. (1996). Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiological Reviews, 60(4), 609–640.
Conrad, R. (1998). Control of methane production in terrestrial ecosystems. In M. O. Andreae & D. S. Schimel (Eds.), Exchange of trace gases between terrestrial ecosystems and the atmosphere (pp. 39–58). Chichester: Wiley.
Conrad, R. (1999). Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments. FEMS Microbiology Ecology, 28(3), 193–202.
Ding, W., Zhang, Y., & Cai, Z. (2010). Impact of permanent inundation on methane emissions from a Spartina alterniflora coastal salt marsh. Atmospheric Environment, 44(32), 3894–3900.
Fetzer, S., Bak, F., & Conrad, R. (1993). Sensitivity of methanogenic bacteria from paddy soil to oxygen and desiccation. FEMS Microbiology Ecology, 12(2), 107–115.
Forland, E. J., Benestad, R., Hanssen-Bauer, I., Haugen, J. E., & Skaugen, T. E. (2011). Temperature and precipitation development at Svalbard 1900–2100. Advances in Meteorology,. doi:10.1155/2011/175296.
Freeman, C., Liska, G., Ostle, N. J., Lock, M. A., Hughes, S., Reynolds, B., et al. (1997). Enzymes and biogeochemical cycling in wetlands during a simulated drought. Biogeochemistry, 39(2), 177–187.
Freeman, C., Ostle, N., & Kang, H. (2001). An enzymic ‘latch’ on a global carbon store: A shortage of oxygen locks up carbon in peatlands by restraining a single enzymes. Nature, 409(6817), 149.
Frenzel, P., & Karofeld, E. (2000). CH4 emission from a hollow-ridge complex in a raised bog: The role of CH4 production and oxidation. Biogeochemistry, 51(1), 91–112.
Graham, E. B., Wieder, W. R., Leff, J. W., Weintraub, S. R., Townsend, A. R., Cleveland, C. C., et al. (2014). Do we need to understand microbial communities to predict ecosystem function? A comparison of statistical models of nitrogen cycling processes. Soil Biology and Biochemistry, 68, 279–282.
Hales, B. A., Edwards, C., Ritchie, D. A., Hall, G., Pickup, R. W., & Saunders, J. R. (1996). Isolation and identification of methanogen-specific DNA from blanket bog peat by PCR amplification and sequence analysis. Applied and Environmental Microbiology, 62(2), 668–675.
Heid, C. A., Stevens, J., Livak, K. J., & Williams, P. M. (1996). Real time quantitative PCR. Genome Research, 6(10), 986–994.
Kim, S. Y., Freeman, C., Fenner, N., & Kang, H. (2012). Functional and structural responses of bacterial and methanogen communities to 3-year warming incubation in different depths of peat mire. Applied Soil Ecology, 57, 23–30.
Kirschke, S., Bousquet, P., Ciais, P., Saunois, M., Canadell, J. G., Dlugokencky, E. J., et al. (2013). Three decades of global methane sources and sinks. Nature Geoscience,. doi:10.1038/ngeo1955.
Lelieveld, J. O. S., Crutzen, P. J., & Dentener, F. J. (1998). Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B, 50(2), 128–150.
Liu, D. Y., Ding, W. X., Jia, Z. J., & Cai, Z. C. (2011). Relation between methanogenic archaea and methane production potential in selected natural wetland ecosystems across China. Biogeosciences, 8(2), 329–338.
Liu, D., Ding, W., Jia, Z., & Cai, Z. (2012). The impact of dissolved organic carbon on the spatial variability of methanogenic archaea communities in natural wetland ecosystems across China. Applied Microbiology and Biotechnology, 96(1), 253–263.
Mah, R., & Smith, M. (1981). The Methanogenic Bacteria. In M. Starr, H. Stolp, H. Trüper, A. Balows, & H. Schlegel (Eds.), The Prokaryotes (pp. 948–977). Berlin: Springer.
Melling, L., Hatano, R., & Kah, J. G. (2005). Methane fluxes from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Biology and Biochemistry, 37(8), 1445–1453.
Morrissey, E. M., Berrier, D. J., Neubauer, S. C., & Franklin, R. B. (2014). Using microbial communities and extracellular enzymes to link soil organic matter characteristics to greenhouse gas production in a tidal freshwater wetland. Biogeochemistry, 117(2–3), 473–490.
Nauta, A. L., Heijmans, M. M. P. D., Blok, D., Limpens, J., Elberling, B., Gallagher, A., et al. (2015). Permafrost collapse after shrub removal shifts tundra ecosystem to a methane source. Nature Climate Change, 5(1), 67–70.
Prescott, C. E. (2010). Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry, 101(1), 133–149.
Reiche, M., Gleixner, G., & Küsel, K. (2010). Effect of peat quality on microbial greenhouse gas formation in an acidic fen. Biogeosciences, 7(1), 187–198.
Rigby, M., Prinn, R. G., Fraser, P. J., Simmonds, P. G., Langenfelds, R. L., Huang, J., et al. (2008). Renewed growth of atmospheric methane. Geophysical Research Letters, 35(22), L22805.
Saarnio, S., Alm, J., Martikainen, P. J., & Silvola, J. (1998). Effects of raised CO2 on potential CH4 production and oxidation in, and CH4 emission from, a boreal mire. Journal of Ecology, 86(2), 261–268.
Shannon, R. D., & White, J. R. (1996). The effects of spatial and temporal variations in acetate and sulfate on methane cycling in two Michigan peatlands. Limnology and Oceanography, 41(3), 435–443.
Shi, W., Dell, E., Bowman, D., & Iyyemperumal, K. (2006). Soil enzyme activities and organic matter composition in a turfgrass chronosequence. Plant and Soil, 288(1–2), 285–296.
Steinberg, L. M., & Regan, J. M. (2008). Phylogenetic comparison of the methanogenic communities from an acidic, oligotrophic fen and an anaerobic digester treating municipal wastewater sludge. Applied and Environmental Microbiology, 74(21), 6663–6671.
Thauer, R. K. (1998). Biochemistry of methanogenesis: a tribute to Marjory Stephenson—1998 Marjory Stephenson Prize Lecture. Microbiology, 144(Pt9), 2377–2406.
Van Bodegom, P. M., Wassmann, R., & Metra-Corton, T. M. (2001). A process-based model for methane emission predictions from flooded rice paddies. Global Biogeochemical Cycles, 15(1), 247–263.
Wang, Z. P., Delaune, R. D., Masscheleyn, P. H., & Patrick, W. H, Jr. (1993). Soil redox and pH effects on methane production in a flooded rice soil. Soil Science Society of America Journal, 57(2), 382–385.
Wang, J. S., Logan, J. A., McElroy, M. B., Duncan, B. N., Megretskaia, I. A., & Yantosca, R. M. (2004). A 3-D model analysis of the slowdown and interannual variability in the methane growth rate from 1988 to 1997. Global Biogeochemical Cycles,. doi:10.1029/2003GB002180.
Westermann, P. (1993). Temperature regulation of methanogenesis in wetlands. Chemosphere, 26(1–4), 321–328.
Wieder, W. R., Bonan, G. B., & Allison, S. D. (2013). Global soil carbon projections are improved by modelling microbial processes. Nature Climate Change, 3(10), 909–912.
Yvon-Durocher, G., Allen, A. P., Bastviken, D., Conrad, R., Gudasz, C., St-Pierre, A., et al. (2014). Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature, 507(7493), 488–491.
Acknowledgments
This study was supported by funds from the National Research Foundations of Korea (2014036440 and 2014050532) and the Rural Development Administration of Korea (PJ009253022014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Lee, SH., Jang, I. et al. Can abundance of methanogen be a good indicator for CH4 flux in soil ecosystems?. Environ Geochem Health 37, 1007–1015 (2015). https://doi.org/10.1007/s10653-015-9729-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9729-5