Summary
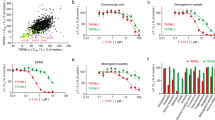
Estrogen receptor-α (ERα) promotes breast cancer, and ER-positive cancer accounts for ~ 80% of breast cancers. This subtype responds positively to hormone/endocrine therapies involving either inhibition of estrogen synthesis or blockade of estrogen action. Carbidopa, a drug used to potentiate the therapeutic efficacy of L-DOPA in Parkinson's disease, is an agonist for aryl hydrocarbon receptor (AhR). Pharmacotherapy in Parkinson’s disease decreases the risk for cancers, including breast cancer. The effects of carbidopa on ER-positive breast cancer were evaluated in cell culture and in mouse xenografts. The assays included cell proliferation, apoptosis, cell migration/invasion, subcellular localization of AhR, proteasomal degradation, and tumor growth in xenografts. Carbidopa decreased proliferation and migration of ER-positive human breast cancer cells in vitro with no significant effect on ER-negative breast cancer cells. Treatment of ER-positive cells with carbidopa promoted nuclear localization of AhR and expression of AhR target genes; it also decreased cellular levels of ERα via proteasomal degradation in an AhR-dependent manner. In vivo, carbidopa suppressed the growth of ER-positive breast cancer cells in mouse xenografts; this was associated with increased apoptosis and decreased cell proliferation. Carbidopa has therapeutic potential for ER-positive breast cancer either as a single agent or in combination with other standard chemotherapies.








Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a Cancer J Clinicians 71:209–249
Salvati A, Gigantino V, Nassa G, Mirici Cappa V, Ventola GM, Cracas DGC, Mastrocinque R, Rizzo F, Tarallo R, Weisz A, Giurato G (2020) Global View of Candidate Therapeutic Target Genes in Hormone-Responsive Breast Cancer. Int J Mol Sci 21
McAndrew NP, Finn RS (2020) Management of ER positive metastatic breast cancer. Semin Oncol 47:270–277
Aggelis V, Johnston SRD (2019) Advances in Endocrine-Based Therapies for Estrogen Receptor-Positive Metastatic Breast Cancer. Drugs 79:1849–1866
Bhardwaj P, Au CC, Benito-Martin A, Ladumor H, Oshchepkova S, Moges R, Brown KA (2019) Estrogens and breast cancer: Mechanisms involved in obesity-related development, growth and progression. J Steroid Biochem Mol Biol 189:161–170
Khatpe AS, Adebayo AK, Herodotou CA, Kumar B, Nakshatri H (2021) Nexus between PI3K/AKT and Estrogen Receptor Signaling in Breast Cancer. Cancers 13
Fan P, Jordan VC (2019) New insights into acquired endocrine resistance of breast cancer. Cancer drug resistance 2:198–209
Ali S, Coombes RC (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2:101–112
de Leeuw R, Neefjes J, Michalides R (2011) A role for estrogen receptor phosphorylation in the resistance to tamoxifen. Int J Breast Cancer 2011:232435
Pratima S, Karnik SK, Liu X-P, Thomas Budd G, Bukowski RM (1994) Estrogen Receptor Mutations in Tamoxifen-resistant Breast Cancer. Cancer Res 54:349–353
Seeberger LC, Hauser RA (2009) Levodopa/carbidopa/entacapone in Parkinson’s disease. Expert Rev Neurother 9:929–940
Bajaj A, Driver JA, Schernhammer ES (2010) Parkinson’s disease and cancer risk: a systematic review and meta-analysis. Cancer causes & control : CCC 21:697–707
Nikolaou V, Stratigos AJ (2014) Emerging trends in the epidemiology of melanoma. Br J Dermatol 170:11–19
Wirdefeldt K, Weibull CE, Chen H, Kamel F, Lundholm C, Fang F, Ye W (2014) Parkinson’s disease and cancer: A register-based family study. Am J Epidemiol 179:85–94
Siple JF, Schneider DC, Wanlass WA, Rosenblatt BK (2000) Levodopa therapy and the risk of malignant melanoma. Ann Pharmacother 34:382–385
Zanetti R, Loria D, Rosso S (2006) Melanoma, Parkinson’s disease and levodopa: causal or spurious link? A review of the literature. Melanoma Res 16:201–206
Ogura J, Miyauchi S, Shimono K, Yang S, Gonchigar S, Ganapathy V, Bhutia YD (2017) Carbidopa is an activator of aryl hydrocarbon receptor with potential for cancer therapy. Biochem J 474:3391–3402
Kawajiri K, Fujii-Kuriyama Y (2017) The aryl hydrocarbon receptor: a multifunctional chemical sensor for host defense and homeostatic maintenance. Exp Anim 66:75–89
Lamorte S, Shinde R, McGaha TL (2021) Nuclear receptors, the aryl hydrocarbon receptor, and macrophage function. Mol Aspects Med 78:100942
Sivaprakasam S, Bhutia YD, Ramachandran S, Ganapathy V (2017) Cell-Surface and Nuclear Receptors in the Colon as Targets for Bacterial Metabolites and Its Relevance to Colon Health. Nutrients 9
Kolluri SK, Jin UH, Safe S (2017) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch Toxicol 91:2497–2513
Safe S, Lee SO, Jin UH (2013) Role of the aryl hydrocarbon receptor in carcinogenesis and potential as a drug target. Toxicol Sci 135:1–16
O’Donnell EF, Koch DC, Bisson WH, Jang HS, Kolluri SK (2014) The aryl hydrocarbon receptor mediates raloxifene-induced apoptosis in estrogen receptor-negative hepatoma and breast cancer cells. Cell Death Dis 5:e1038
Baker JR, Sakoff JA, McCluskey A (2020) The aryl hydrocarbon receptor (AhR) as a breast cancer drug target. Med Res Rev 40:972–1001
Vogel CFA, Lazennec G, Kado SY, Dahlem C, He Y, Castaneda A, Ishihara Y, Vogeley C, Rossi A, Haarmann-Stemmann T, Jugan J, Mori H, Borowsky AD, La Merrill MA, Sweeney C (2021) Targeting the Aryl Hydrocarbon Receptor Signaling Pathway in Breast Cancer Development. Front Immunol 12:625346
Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S (2007) Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature 446:562–566
Luecke-Johansson S, Gralla M, Rundqvist H, Ho JC, Johnson RS, Gradin K, and Poellinger L (2017) A Molecular Mechanism To Switch the Aryl Hydrocarbon Receptor from a Transcription Factor to an E3 Ubiquitin Ligase. Mol Cellular Biol 37
Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S (2003) The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol Cell Biol 23:1843–1855
Ohtake F, Fujii-Kuriyama Y, Kato S (2009) AhR acts as an E3 ubiquitin ligase to modulate steroid receptor functions. Biochem Pharmacol 77:474–484
Salzano M, Marabotti A, Milanesi L, Facchiano A (2011) Human aryl-hydrocarbon receptor and its interaction with dioxin and physiological ligands investigated by molecular modelling and docking simulations. Biochem Biophys Res Commun 413:176–181
Casalegno M, Raos G, Sello G (2021) Identification of viable TCDD access pathways to human AhR PAS-B ligand binding domain. J Mol Graph Model 105:107886
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461
Wafa LA, Cheng H, Plaa N, Ghaidi F, Fukumoto T, Fazli L, Gleave ME, Cox ME, Rennie PS (2012) Carbidopa abrogates L-dopa decarboxylase coactivation of the androgen receptor and delays prostate tumor progression. Int J Cancer 130:2835–2844
Petrulis JR, Perdew GH (2002) The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact 141:25–40
Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH (2008) The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr 18:207–250
Hoy SM (2019) Levodopa/Carbidopa Enteral Suspension: A Review in Advanced Parkinson’s Disease. Drugs 79:1709–1718
Chen Z, Cai A, Zheng H, Huang H, Sun R, Cui X, Ye W, Yao Q, Chen R, Kou L (2020) Carbidopa suppresses prostate cancer via aryl hydrocarbon receptor-mediated ubiquitination and degradation of androgen receptor. Oncogenesis 9:49
Gilbert JA, LMF, and Matthew M. Ames, (2000) The Aromatic-L-Amino Acid Decarboxylase Inhibitor Carbidopa Is Selectively Cytotoxic to Human Pulmonary Carcinoid and Small Cell Lung Carcinoma Cells. Clin Cancer Res 6:4365–4372
Vermeij JD, Winogrodzka A, Trip J, Weber WE (2009) Parkinson’s disease, levodopa-use and the risk of melanoma. Parkinsonism Relat Disord 15:551–553
D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D’Alessandro A, Hansen KC, Richer JK (2015) A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res 75:4651–4664
Chen I, Hsieh T, Thomas T, Safe S (2001) Identification of estrogen-induced genes downregulated by AhR agonists in MCF-7 breast cancer cells using suppression subtractive hybridization. Gene 262:207–214
Brod LS, Aldred JL, Nutt JG (2012) Are high doses of carbidopa a concern? A randomized, clinical trial in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society 27:750–753
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22:659–661
(2005) Guidance for Industry. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers (U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Rockville, MD
Funding
This research was supported by Zhejiang Province Natural Science Foundation (LQ19H300001, LYY21H300007), Wenzhou Municipal Science and Technology Bureau (Y20190174), and Excellent Young Scientist Training Program fund from Wenzhou Medical University.
Author information
Authors and Affiliations
Contributions
Conceived the study and designed the experiment: Longfa Kou, Maoping Chu, Ruijie Chen and Vadivel Ganapathy. Performed the experiments: Zhiwei Chen, Xing Xia, Heyan Chen, Huirong Huang, Xingsi An and Sun Meng. Contributed new reagents or analytic tools: Yangzom D. Bhutia, Qing Yao, Kwonseop Kim and Hailin Zhang. Analyzed the data: Zhiwei Chen, Xing Xia and Heyan Chen.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The animal studies were reviewed and approved by the Institutional Animal Care and Use Committee (approval number: wydw2019-0704).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Xia, X., Chen, H. et al. Carbidopa suppresses estrogen receptor-positive breast cancer via AhR-mediated proteasomal degradation of ERα. Invest New Drugs 40, 1216–1230 (2022). https://doi.org/10.1007/s10637-022-01289-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-022-01289-5