Summary
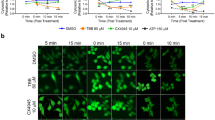
Chaetoglobosin K (ChK) is a natural product that has been shown to promote F-actin capping, inhibit growth, arrest cell cycle G2 phase, and induce apoptosis. ChK also has been shown to downregulate two important kinases involved in oncogenic pathways, Akt and JNK. This report investigates how ChK is involved in the receptor tyrosine kinase pathway (RTK/PI3K/mTORC2/Akt) to the centrally located protein kinase, Akt. Studies have reported that ChK does not inhibit PI3K comparable to wortmannin and does not affect PDK1 activation. PDK1 is responsible for phosphorylation on Akt T308, while mTORC2 phosphorylates Akt S473. Yet, Akt’s two activation sites, T308 and S473, are known to be affected by ChK treatment. It was our hypothesis that ChK acts on the mTORC2 complex to inhibit the phosphorylation seen at Akt S473. This inhibition at mTORC2 should decrease phosphorylation at both these proteins, Akt and mTORC2 complex, compared to a known mTOR specific inhibitor, Torin1. Human lung adenocarcinoma H1299 and H2009 cells were treated with IGF-1 or calyculin A to increase phosphorylation at complex mTORC2 and Akt. Pretreatment with ChK was able to significantly decrease phosphorylation at Akt S473 similarly to Torin1 with either IGF-1 or calyculin A treatment. Moreover, the autophosphorylation site on complex mTORC2, S2481, was also significantly reduced with ChK pretreatment, similar to Torin1. This is the first report to illustrate that ChK has a significant effect at mTORC2 S2481 and Akt S473 comparable to Torin1, indicating that it may be a mTOR inhibitor.







Similar content being viewed by others
References
Wang Y, Schmid-Bindert G, Zhou C (2012) Erlotinib in the treatment of advanced non-small cell lung cancer: an update for clinicians. Ther Adv Med Oncol 4:19–29
tarceva_prescribing.pdf [Internet]. [cited 2017 Dec 6]. Available from: https://www.gene.com/download/pdf/tarceva_prescribing.pdf
Blanco R, Iwakawa R, Tang M, Kohno T, Angulo B, Pio R, Montuenga LM, Minna JD, Yokota J, Sanchez-Cespedes M (2009) A gene-alteration profile of human lung Cancer cell lines. Hum Mutat 30:1199–1206
Shah A, Mangaonkar A (2015) Idelalisib: a novel PI3Kδ inhibitor for chronic lymphocytic leukemia. Ann Pharmacother 49:1162–1170
Markham A (2017) Copanlisib: first global approval. Drugs 77:2057–2062
Vu T, Claret FX. Trastuzumab: Updated Mechanisms of Action and Resistance in Breast Cancer. Front Oncol [Internet]. 2012 [cited 2017 Dec 7]; 2. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3376449/
Yang W-L, Wu C-Y, Wu J, Lin H-K (2010) Regulation of Akt signaling activation by ubiquitination. Cell Cycle 9:486–497
Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E (2008) The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 27:1932–1943
Mora A, Komander D, van Aalten DMF, Alessi DR (2004) PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15:161–170
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (2005) Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR complex. Science 307:1098–1101
Jordan NJ, Dutkowski CM, Barrow D, Mottram HJ, Hutcheson IR, Nicholson RI et al (2014) Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res 16:3370
Liu Q, Xu C, Kirubakaran S, Zhang X, Hur W, Liu Y, Kwiatkowski NP, Wang J, Westover KD, Gao P, Ercan D, Niepel M, Thoreen CC, Kang SA, Patricelli MP, Wang Y, Tupper T, Altabef A, Kawamura H, Held KD, Chou DM, Elledge SJ, Janne PA, Wong KK, Sabatini DM, Gray NS (2013) Characterization of Torin2, an ATP-competitive inhibitor of mTOR, ATM, and ATR. Cancer Res 73:2574–2586
Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Abraham RT (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15:5256–5267
Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC (2010) mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285:7866–7879
Hart S, Novotny-Diermayr V, Goh KC, Williams M, Tan YC, Ong LC, Cheong A, Ng BK, Amalini C, Madan B, Nagaraj H, Jayaraman R, Pasha KM, Ethirajulu K, Chng WJ, Mustafa N, Goh BC, Benes C, McDermott U, Garnett M, Dymock B, Wood JM (2013) VS-5584, a novel and highly selective PI3K/mTOR kinase inhibitor for the treatment of Cancer. Mol Cancer Ther 12:151–161
Yuan J, Mehta PP, Yin M-J, Sun S, Zou A, Chen J, Rafidi K, Feng Z, Nickel J, Engebretsen J, Hallin J, Blasina A, Zhang E, Nguyen L, Sun M, Vogt PK, McHarg A, Cheng H, Christensen JG, Kan JLC, Bagrodia S (2011) PF-04691502, a potent and selective Oral inhibitor of PI3K and mTOR kinases with antitumor activity. Mol Cancer Ther 10:2189–2199
del CJM, Birrer M, Davis C, Fujiwara K, Gollerkeri A, Gore M et al (2016) A randomized phase II non-comparative study of PF-04691502 and gedatolisib (PF-05212384) in patients with recurrent endometrial cancer. Gynecol Oncol 142:62–69
Ali A, Sidorova TS, Matesic DF (2013) Dual modulation of JNK and Akt signaling pathways by chaetoglobosin K in human lung carcinoma and ras-transformed epithelial cells. Investig New Drugs 31:525–534
Matesic DF, Villio KN, Folse SL, Garcia EL, Cutler SJ, Cutler HG (2006) Inhibition of cytokinesis and akt phosphorylation by chaetoglobosin K in ras-transformed epithelial cells. Cancer Chemother Pharmacol 57:741–754
Li B, Gao Y, Rankin GO, Rojanasakul Y, Cutler SJ, Tu Y, Chen YC (2015) Chaetoglobosin K induces apoptosis and G2 cell cycle arrest through p53-dependent pathway in cisplatin-resistant ovarian cancer cells. Cancer Lett 356:418–433
Tikoo A, Cutler H, Lo SH, Chen LB, Maruta H (1999) Treatment of Ras-induced cancers by the F-actin cappers tensin and chaetoglobosin K, in combination with the caspase-1 inhibitor N1445. Cancer J Sci Am 5:293–300
Luo H, Li B, Li Z, Cutler SJ, Rankin GO, Chen YC (2013) Chaetoglobosin K inhibits tumor angiogenesis through downregulation of vascular epithelial growth factor-binding hypoxia-inducible factor 1α. Anti-Cancer Drugs 24:715–724
Matesic D, Ali A, Sidorova T, Burns T (2014) A cell-cell communication marker for identifying targeted tumor therapies. Curr Bioact Compd 9:255–262
Ikenoue T, Inoki K, Yang Q, Zhou X, Guan K-L (2008) Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 27:1919–1931
Vadlakonda L, Dash A, Pasupuleti M, Anil Kumar K, Reddanna P. The Paradox of Akt-mTOR Interactions. Front Oncol [Internet]. 2013 [cited 2016 Dec 15]; 3. Available from: http://journal.frontiersin.org/article/10.3389/fonc.2013.00165/abstract
Feldman ME, Shokat KM (2010) New inhibitors of the PI3K-Akt-mTOR pathway: insights into mTOR signaling from a new generation of Tor kinase domain inhibitors (TORKinibs). Curr Top Microbiol Immunol 347:241–262
Sparks CA, Guertin DA (2010) Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene 29:3733–3744
Copp J, Manning G, Hunter T (2009) TORC-specific Phosphorylation of mammalian target of rapamycin (mTOR): Phospho-Ser2481 is a marker for intact mTOR Signaling complex 2. Cancer Res 69:1821–1827
Rosner M, Siegel N, Valli A, Fuchs C, Hengstschläger M (2010) mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 38:223–228
Peterson RT, Beal PA, Comb MJ, Schreiber SL (2000) FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275:7416–7423
Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS (2009) An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284:8023–8032
Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N (2011) mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT Signaling. Cancer Discov 1:248–259
O’Reilly KE (2006) mTOR inhibition induces upstream receptor tyrosine kinase Signaling and activates Akt. Cancer Res 66:1500–1508
Cutler HG, Crumley FG, Cox RH, Cole RJ, Dorner JW, Springer JP, Latterell FM, Thean JE, Rossi AE (1980) Chaetoglobosin K: a new plant growth inhibitor and toxin from Diplodia macrospora. J Agric Food Chem 28:139–142
Moore SF, Hunter RW, Hers I (2011) mTORC2 protein-mediated protein kinase B (Akt) serine 473 Phosphorylation is not required for Akt1 activity in human platelets. J Biol Chem 286:24553–24560
Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137:873–886
Liao Y, Hung M-C (2010) Physiological regulation of Akt activity and stability. Am J Transl Res 2:19–42
Pozuelo-Rubio M, Leslie NR, Murphy J, MacKintosh C (2010) Mechanism of activation of PKB/Akt by the protein phosphatase inhibitor Calyculin a. Cell Biochem Biophys 58:147–156
Wander SA, Hennessy BT, Slingerland JM (2011) Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest 121:1231–1241
Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, Hur W, Zhang J, Sim T, Sabatini DM, Gray NS (2010) Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 53:7146–7155
Zheng Y, Jiang Y (2015) mTOR inhibitors at a glance. Mol Cell Pharmacol 7:15–20
Watanabe R, Wei L, Huang J. mTOR Signaling, Function, Novel Inhibitors, and Therapeutic Targets J Nucl Med [Internet]. 2011 [cited 2016 Dec 15]; Available from: http://jnm.snmjournals.org/cgi/doi/10.2967/jnumed.111.089623
Barlow AD, Xie J, Moore CE, Campbell SC, Shaw JAM, Nicholson ML, Herbert TP (2012) Rapamycin toxicity in MIN6 cells and rat and human islets is mediated by the inhibition of mTOR complex 2 (mTORC2). Diabetologia 55:1355–1365
Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000 Research [Internet]. 2016 [cited 2017 Dec 7];5. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5007757/
Funding
This work was supported by the Mercer University College of Pharmacy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Blair P Curless declares that he has no conflict of interest. Nne E Uko declares that she has no conflict of interest. Diane F Matesic declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Curless, B.P., Uko, N.E. & Matesic, D.F. Modulator of the PI3K/Akt oncogenic pathway affects mTOR complex 2 in human adenocarcinoma cells. Invest New Drugs 37, 902–911 (2019). https://doi.org/10.1007/s10637-018-0705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0705-7