Summary
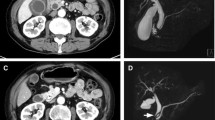
Background Nivolumab demonstrates promising efficacy for the treatment of non-small cell lung cancer and other malignancies. The clinical benefit of nivolumab, however, may be hampered by specific immune-related adverse events (irAEs), and little is known regarding nivolumab-related cholangitis. Methods A computerized search of our clinical database identified 3 metastatic non-small cell lung cancer patients with nivolumab-related cholangitis. All patients were treated with intravenous nivolumab monotherapy (3.0 mg/kg) every 2 weeks until disease progression or irAEs occurred. Clinical data regarding the duration of nivolumab treatment, symptoms, laboratory abnormalities, pathological findings of liver parenchyma biopsy specimens, and management of nivolumab-related cholangitis were analyzed. Results Our analysis revealed that nivolumab-related cholangitis was characterized by (1) localized extrahepatic bile duct dilation without obstruction; (2) diffuse hypertrophy of the extrahepatic bile duct wall; (3) a dominant increase in the biliary tract enzymes alkaline phosphatase and gamma-glutamyl transpeptidase relative to the hepatic enzymes aspartate and alanine aminotransferase; (4) normal or reduced levels of the serum immunological markers antinuclear antibody, antimitochondrial antibody, smooth muscle antibody, and immunoglobulin G4; (5) the pathological finding of biliary tract cluster of differentiation 8-positive T cell infiltration from liver biopsy; and (6) a moderate to poor response to steroid therapy. Conclusions Nivolumab-related cholangitis is associated with distinct imaging and clinicopathological features that distinguish it from acute cholangitis of common etiologies and other immune-related cholangitis. Further studies are warranted to establish the optimal management of patients with this irAE.




Similar content being viewed by others
References
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454. doi:10.1056/NEJMoa1200690
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465. doi:10.1056/NEJMoa1200694
Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030. doi:10.1200/JCO.2013.53.0105
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562. doi:10.1038/nature13904
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. doi:10.1038/nature14011
Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369:134–144. doi:10.1056/NEJMoa1305133
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311–319. doi:10.1056/NEJMao1411087
Rizvi NA, Mazières J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E, Mennecier B, Otterson GA, Campos LT, Gandara DR, Levy BP, Nair SG, Zalcman G, Wolf J, Souquet PJ, Baldini E, Cappuzzo F, Chouaid C, Dowlati A, Sanborn R, Lopez-Chavez A, Grohe C, Huber RM, Harbison CT, Baudelet C, Lestini BJ, Ramalingam SS (2015) Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 16:257–265. doi:10.1016/S1470-2045(15)70054-9
Friedman CF, Proverbs-Singh TA, Postow MA (2016) Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2:1346–1353. doi:10.1001/jamaoncol.2016.1051
Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T (2001) Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319–322
Chen L (2004) Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 4:336–347
Nishimura H, Nose M, Hiai H, Minato N, Honjo T (1999) Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11:141–151
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123–135. doi:10.1056/NEJMoa1504627
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip EE, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced Nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Gelsomino F, Vitale G, D’Errico A, Bertuzzi C, Andreone P, Ardizzoni A (2016) Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol. doi:10.1093/annonc/mdw649
Björnsson E, Chari ST, Smyrk TC, Lindor K (2007) Immunoglobulin G4 associated cholangitis: description of an emerging clinical entity based on review of the literature. Hepatology 45:1547–1554
Zen Y, Harada K, Sasaki M, Sato Y, Tsuneyama K, Haratake J, Kurumaya H, Katayanagi K, Masuda S, Niwa H, Morimoto H, Miwa A, Uchiyama A, Portmann BC, Nakanuma Y (2004) IgG4-related sclerosing cholangitis with and without hepatic inflammatory pseudotumor, and sclerosing pancreatitis-associated sclerosing cholangitis: do they belong to a spectrum of sclerosing pancreatitis? Am J Surg Pathol 28:1193–1203
Wu CT, Davis PA, Luketic VA, Gershwin ME (2004) A review of the physiological and immunological functions of biliary epithelial cells: targets for primary biliary cirrhosis, primary sclerosing cholangitis and drug-induced ductopenias. Clin Dev Immunol 11:205–213
Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME (1999) The immunobiology of bile and biliary epithelium. Hepatology 30:351–357
Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A, Handa M, Ishibashi H, Gershwin ME, Harada M (2005) Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology 41:151–159
Chen L, Han X (2015) Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest 125:3384–3391
Acknowledgements
We wish to thank to our colleagues in the Departments of Medical Oncology, Gastroenterology and Hepatology, and Pathology at Kindai University Faculty of Medicine (Osaka, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hisato Kawakami declares that he has no conflict of interest. Junko Tanizaki declares that she has no conflict of interest. Kaoru Tanaka declares that he has no conflict of interest. Koji Haratani declares that he has no conflict of interest. Hidetoshi Hayashi declares that he has no conflict of interest. Masayuki Takeda declares that he has no conflict of interest. Ken Kamata declares that he has no conflict of interest. Mamoru Takenaka declares that he has no conflict of interest. Masatomo Kimura declares that he has no conflict of interest. Takaaki Chikugo declares that he has no conflict of interest. Takao Sato declares that he has no conflict of interest. Masatoshi Kudo declares that he has no conflict of interest. Akihiko Ito declares that he has no conflict of interest. Kazuhiko Nakagawa declares that he has no conflict of interest.
Funding
None declared.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kawakami, H., Tanizaki, J., Tanaka, K. et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs 35, 529–536 (2017). https://doi.org/10.1007/s10637-017-0453-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0453-0