Summary
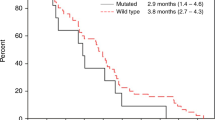
Background KRAS mutations are clinically important predictors of resistance to EGFR-directed therapies in colorectal cancer (CRC). Oncogenic activation of the RAS/RAF/MEK/ERK signaling cascade mediates proliferation independent of growth factor signaling. We hypothesized that targeting MEK with selumetinib could overcome resistance to cetuximab in KRAS mutant CRC. Methods A phase I study (NCT01287130) was undertaken to determine the tolerability, and pharmacokinetic profiles of the combination of selumetinib and cetuximab, with an expanded cohort in KRAS-mutant CRC. Results 15 patients were treated in the dose escalation cohort and 18 patients were treated in the expansion cohort. Two dose-limiting toxicities were observed. One grade 3 acneiform rash and one grade 4 hypomagnesemia occurred. The most common grade 1 and 2 adverse events included rash, nausea/vomiting, diarrhea, and fatigue. The maximum tolerated dose was established at selumetinib 75 mg PO BID and cetuximab 250 mg/m2 weekly following a 400 mg/m2 load. Best clinical response in the dose escalation group included 1 unconfirmed partial response in a patient with CRC and stable disease (SD) in 5 patients (1 squamous cell carcinoma of the tonsil, 1 non-small cell lung cancer, and 3 CRC), and in the KRAS-mutant CRC dose expansion cohort, of the 14 patients who were evaluable for response, 5 patients had SD and 9 patients had progressive disease. Conclusions The combination of selumetinib and cetuximab is safe and well tolerated. Minimal anti-tumor activity was observed in KRAS-mutant refractory metastatic CRC. Further investigations might be warranted in other cancer subtypes.
Similar content being viewed by others
References
Ciardiello F, Tortora G (2008) EGFR antagonists in cancer treatment. N Engl J Med 358:1160–1174
Karapetis CS, Khambata-Ford S, Jonker DJ et al (2008) K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 359:1757–1765
Benvenuti S, Sartore-Bianchi A, Nicolantonio DI F et al (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643–2648
Souglakos J, Philips J, Wang R et al (2009) Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer 101:465–472
Yokota T, Ura T, Shibata N et al (2011) BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer 104:856–862
Nicolantonio DI F, Martini M, Molinari F et al (2008) Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 26:5705–5712
Yeh TC, Marsh V, Bernat BA et al (2007) Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res 13:1576–1583
Davies BR, Logie A, Mckay JS et al (2007) AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther 6:2209–2219
Adjei AA, Cohen RB, Franklin W et al (2008) Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 26:2139–2146
Banerji U, Camidge DR, Verheul HM et al (2010) The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res 16:1613–1623
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (Version 1.1). Eur J Cancer 45:228–247
Ikesue H, Vermeulen LC, Hoke R et al (2010) Stability of cetuximab and panitumumab in glass vials and polyvinyl chloride bags. Am J Health Syst Pharm 67:223–226
Ballif BA, Blenis J (2001) Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ 12:397–408
Corcoran RB, Atreya CE, Falchook GS et al (2014) Phase 1-2 trial of the BRAF inhibitor dabrafenib (D) plus MEK inhibitor trametinib (T) in BRAF V600 mutant colorectal cancer (CRC): updated efficacy and biomarker analysis. J Clin Oncol 32:5s (suppl; abstr 3517)
Bendell JC, Atreya CE, Theirry A et al Efficacy and tolerability in an open-label phase I/II study of MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D), and anti-EGFR antibody panitumumab (P) in combination in patients (pts) with BRAF V600E mutated colorectal cancer (CRC). J Clin Oncol 32:5s (suppl; abstr 3515)
Van Geel R, Elez E, Bendell JC, et al (2014) Phase I study of the selective BRAFV600 inhibitor encorafenib (LGX818) combined with cetuximab and with or without the α-Specific PI3K inhibitor BYL719 in patients with advanced BRAF-mutant colorectal cancer. J Clin Oncol 32:5s (suppl; abstr 3514)
Hong DS, Van Karlyle M, Fu S, et al (2014) Phase 1B study of vemurafenib in combination with irinotecan and cetuximab in patients with BRAF-mutated advanced cancers and metastatic colorectal cancer. J Clin Oncol 32:5s (suppl; abstr 3516)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by grants from the National Cancer Institute: U01 CA062491to Glenn Liu and P30 CA014520 to University of Wisconsin–Madison Carbone Cancer Center. We gratefully acknowledge the support of AstraZeneca who provided the selumetinib for this clinical trial.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Deming, D.A., Cavalcante, L.L., Lubner, S.J. et al. A phase I study of selumetinib (AZD6244/ARRY-142866), a MEK1/2 inhibitor, in combination with cetuximab in refractory solid tumors and KRAS mutant colorectal cancer. Invest New Drugs 34, 168–175 (2016). https://doi.org/10.1007/s10637-015-0314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0314-7