Abstract
Aim
This study was to investigate the effects and mechanisms of miR-362-3p on regulation of gastric cancer (GC) cell metastasis potential.
Methods
We detected miR-362-3p level in GC and adjacent normal tissues and investigated the relationship with clinicopathological factors. Next, we analyzed the level of miR-362-3p expression and CD82 in different differentiated GC cells compared with a normal gastric mucosa cell by RT-PCR and Western blot. Dual-luciferase reporter assay and Western blot confirmed a direct interaction between miR-362-3p and CD82 3′UTR. After miR-362-3p and CD82 were silenced in GC cells, we compared the transfected GC cells migration and invasion capacity by transwell assay. In addition, we detected the effects on cells angiogenesis by tube formation assay. Western blot was used to detect the impact of CD82 and miR-362-3p on epithelial-to-mesenchymal transition markers in treated GC cells.
Results
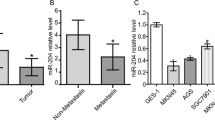
Level of miR-362-3p expression was much higher in GC cells than in normal gastric mucosa cell, and miR-362-3p expression negatively correlated with CD82 mRNA expression in these cell lines. Furthermore, miR-362-3p expression inhibited GC cell metastasis capacity by suppression of CD82 expression. Level of miR-362-3p may mediate E-cadherin, N-cadherin, and vimentin expression in GC cells.
Conclusion
This study illuminated that downregulation of miR-362-3p along with the upregulation of CD82 in GC cells resulted in the inhibition of GC migration and invasion. Thus, our results suggested that miR-362-3p or CD82 can be exploited as a new potential target for control of GC in the future.





Similar content being viewed by others
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Dong JT, Suzuki H, Pin SS, et al. Down-regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res. 1996;56:4387–4390.
Shiwu WU, Lan Y, Wenqing S, et al. Expression and clinical significance of CD82/KAI1 and E-cadherin in non-small cell lung cancer. Arch Iran Med. 2012;15:707–712.
Ma ZB, Li K, Wang J, Guo GH. Role of KAI1/CD82 polymorphisms in colon cancer risk in Han Chinese population. Med Oncol. 2013;30:668.
Zhang B, Liu W, Li L, et al. KAI1/CD82 and cyclin D1 as biomarkers of invasion, metastasis and prognosis of laryngeal squamous cell carcinoma. Int J Clin Exp Pathol.. 2013;6:1060–1067.
Kwon HJ, Min SY, Park MJ, et al. Expression of CD9 and CD82 in clear cell renal cell carcinoma and its clinical significance. Pathol Res Pract. 2014;210:285–290.
Yao Y, Suo AL, Li ZF, et al. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–970.
Li X, Zhang Y, Zhang H, et al. MiRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res.. 2011;9:824–833.
Xia JT, Chen LZ, Jian WH, et al. MicroRNA-362 induces cell proliferation and apoptosis resistance in gastric cancer by activation of NF-κB signaling. J Transl Med. 2014;12:33.
Yesildal F, Aydin F, Deveci S, et al. Aspartame induces angiogenesis in vitro and in vivo models. Hum Exp Toxicol. 2014. (Epub ahead of print). doi:10.1177/0960327114537535.
Shi GM, Ke AW, Zhou J, et al. CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology. 2010;52:183–196.
Dai W, Wang C, Wang F, et al. Anti-miR-197 inhibits migration in HCC cells by targeting KAI 1/CD82. Biochem Biophys Res Commun. 2014;446:541–548.
Xu L, Wang F, Xu XF, et al. Down-regulation of miR-212 expression by DNA hypermethylation in human gastric cancer cells. Med Oncol. 2011;28:S189–S196.
Chen CF, Ridzon DA, Broomer AJ. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408.
Pienta KJ, Robertson BA, Coffey DS, et al. The cancer diaspora: metastasis beyond the seed and soil hypothesis. Clin Cancer Res. 2013;19:5849–5855.
Seyfried TN, Huysentruyt LC. On the origin of cancer metastasis. Crit Rev Oncog. 2013;18:43–73.
Mathot L, Stenninger J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer Sci. 2012;103:626–631.
Samatov TR, Tonevitsky AG, Schumacher U. Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer.. 2013;12:107.
Zheng H, Kang Y. Multilayer control of the EMT master regulators. Oncogene. 2014;33:1755–1763.
Christensen LL, Tobiasen H, Holm A, et al. MiRNA-362-3p induces cell cycle arrest through targeting of E2F1, USF2 and PTPN1 and is associated with recurrence of colorectal cancer. Int J Cancer. 2013;133:67–78.
Schröder HM, Hoffmann SC, Hecker M, et al. The tetraspanin network modulates MT1-MMP cell surface trafficking. Int J Biochem Cell Biol. 2013;45:1133–1144.
Van der Auwera I, Limame R, van Dam P, et al. Integrated miRNA and mRNA expression profiling of the inflammatory breast cancer subtype. Br J Cancer. 2010;103:532–541.
Hiraga R, Kato M, Miyagawa S, et al. Nox4-derived ROS signaling contributes to TGF-β-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431–4438.
Lv H, Liu R, Fu J, et al. Epithelial cell-derived periostin functions as a tumor suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway. Cell Cycle. 2014;13:2962–2974.
Sarkar S, Mandal C, Sangwan R, et al. Coupling G2/M arrest to the Wnt/β-catenin pathway restrains pancreatic adenocarcinoma. Endocr Relat Cancer. 2014;21:113–125.
Duan F, Lin M, Li C, et al. Effects of inhibition of hedgehog signaling on cell growth and migration of uveal melanoma cells. Cancer Biol Ther. 2014;15:544–559.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, QH., Yao, YL., Wu, XY. et al. Anti-miR-362-3p Inhibits Migration and Invasion of Human Gastric Cancer Cells by Its Target CD82. Dig Dis Sci 60, 1967–1976 (2015). https://doi.org/10.1007/s10620-015-3563-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3563-6