Abstract
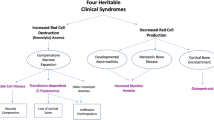
Hematopoietic stem cell transplantation (HSCT) is often the only practical approach to fatal genetic defects. One of the first pathologies which HSCT was applied to was Autosomal Recessive Osteopetrosis (ARO), a rare genetic bone disease in which a deficit in bone resorption by osteoclasts leads to increased bone density and secondary defects. The disease is often lethal early in life unless treated with HSCT. In utero transplantation (IUT) of the oc/oc mouse, reproducing the clinical features of a subset of ARO, has demonstrated that the quality of life and the survival of transplanted animals are greatly improved, suggesting that a similar protocol could be applied to humans. However, recently the dissection of the molecular bases of the disease has shown that ARO is genetically heterogeneous and has revealed the presence of subsets of patients which do not benefit from HSCT. This observation highlights the importance of molecular diagnosing ARO to identify and establish the proper therapies for a better prognosis. In particular, on the basis of experimental results in murine models, efforts should be undertaken to develop approaches such as IUT and new pharmacological strategies.

Similar content being viewed by others
References
Campos-Xavier AB, Casanova JL, Doumaz Y, Feingold J, Munnich A, Cormier-Daire V (2005) Intrafamilial phenotypic variability of osteopetrosis due to chloride channel 7 (CLCN7) mutations. Am J Med Genet A 133A:216–218. doi:10.1002/ajmg.a.30490
Castellano Chiodo D, DiRocco M, Gandolfo C, Morana G, Buzzi D, Rossi A (2007) Neuroimaging findings in malignant infantile osteopetrosis due to OSTM1 mutations. Neuropediatrics 38:154–156. doi:10.1055/s-2007-990267
Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A, Villa A, Vacher J (2003) Grey-lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med 9:399–406. doi:10.1038/nm842
Cleiren E, Bénichou O, Van Hul E, Gram J, Bollerslev J, Singer FR, Beaverson K, Aledo A, Whyte MP, Yoneyama T, de Vernejoul MC, Van Hul W (2001) Albers-Schönberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 10:2861–2867. doi:10.1093/hmg/10.25.2861
Coccia PF, Krivit W, Cervenka J, Clawson C, Kersey JH, Kim TH, Nesbit ME, Ramsay NK, Warkentin PI, Teitelbaum SL, Kahn AJ, Brown DM (1980) Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med 302:701–708
Corbacioglu S, Hönig M, Lahr G, Stöhr S, Berry G, Friedrich W, Schulz AS (2006) Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant 38:547–553. doi:10.1038/sj.bmt.1705485
Corsten MF, Shah K (2008) Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol 9:376–384R. doi:10.1016/S1470-2045(08)70099-8
Driessen GJ, Gerritsen EJ, Fischer A, Fasth A, Hop WC, Veys P, Porta F, Cant A, Steward CG, Vossen JM, Uckan D, Friedrich W (2003) Long-term outcome of haematopoietic stem cell transplantation in autosomal recessive osteopetrosis: an EBMT report. Bone Marrow Transplant 32:657–663. doi:10.1038/sj.bmt.1704194
Flanagan AM, Massey HM, Wilson C, Vellodi A, Horton MA, Steward CG (2002) Macrophage colony-stimulating factor and receptor activator NF-kappaB ligand fail to rescue osteoclast-poor human malignant infantile osteopetrosis in vitro. Bone 30:85–90. doi:10.1016/S8756-3282(01)00656-1
Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A (2000) Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25:343–346. doi:10.1038/77131
Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M, Abinun M, Andolina M, Flanagan A, Horwitz EM, Mihci E, Notarangelo LD, Ramenghi U, Teti A, Van Hove J, Vujic D, Young T, Albertini A, Orchard PJ, Vezzoni P, Villa A (2003) Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res 18:1740–1747. doi:10.1359/jbmr.2003.18.10.1740
Frattini A, Blair HC, Sacco MG, Cerisoli F, Faggioli F, Catò EM, Pangrazio A, Musio A, Rucci F, Sobacchi C, Sharrow AC, Kalla SE, Bruzzone MG, Colombo R, Magli MC, Vezzoni P, Villa A (2005) Rescue of ATPa3-deficient murine malignant osteopetrosis by hematopoietic stem cell transplantation in utero. Proc Natl Acad Sci USA 102:14629–14634. doi:10.1073/pnas.0507637102
Gerritsen EJ, Vossen JM, Fasth A, Friedrich W, Morgan G, Padmos A, Vellodi A, Porras O, O’Meara A, Porta F et al (1994) Bone marrow transplantation for autosomal recessive osteopetrosis. A report from the Working Party on Inborn Errors of the European Bone Marrow Transplantation Group. J Pediatr 125:896–902. doi:10.1016/S0022-3476(05)82004-9
Granero-Molto F, Weis JA, Longobardi L, Spagnoli A (2008) Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 8:255–268. doi:10.1517/14712598.8.3.255
Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A, Moratto D, Mazzolari E, Clayton-Smith J, Orchard P, Coxon FP, Helfrich MH, Crockett JC, Mellis D, Vellodi A, Tezcan I, Notarangelo LD, Rogers MJ, Vezzoni P, Villa A, Frattini A (2008) Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 83:64–76
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann NY Acad Sci 1092:385–396. doi:10.1196/annals.1365.035
Jaing TH, Hou JW, Chen SH, Huang IA, Wang CJ, Lee WI (2006) Successful unrelated mismatched cord blood transplantation in a child with malignant infantile osteopetrosis. Pediatr Transplant 10:629–631. doi:10.1111/j.1399-3046.2006.00537.x
Kim N, Odgren PR, Kim DK, Marks SC Jr, Choi Y (2000) Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci USA 97:10905–10910. doi:10.1073/pnas.200294797
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323. doi:10.1038/16852
Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch TJ, Kubisch C (2000) Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 9:2059–2063. doi:10.1093/hmg/9.13.2059
Kornak U, Kasper D, Bosl MR, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch TJ (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215. doi:10.1016/S0092-8674(01)00206-9
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shaloub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176. doi:10.1016/S0092-8674(00)81569-X
Lange PF, Wartosch L, Jentsch TJ, Fuhrmann JC (2006) ClC-7 requires Ostm1 as a beta-subunit to support bone resorption and lysosomal function. Nature 440:220–223. doi:10.1038/nature04535
Liu G, Shu C, Cui L, Liu W, Cao Y (2008) Tissue-engineered bone formation with cryopreserved human bone marrow mesenchymal stem cells. Cryobiology 56:209–215. doi:10.1016/j.cryobiol.2008.02.008
Maranda B, Chabot G, Décarie JC, Pata M, Azeddine B, Moreau A, Vacher J (2008) Clinical and cellular manifestations of OSTM1-related infantile osteopetrosis. J Bone Miner Res 23:296–300. doi:10.1359/jbmr.071015
Meadows NA, Sharma SM, Faulkner GJ, Ostrowski MC, Hume DA, Cassady AI (2007) The expression of chloride channel 7 (ClCN7) and OSTM1 in osteoclasts is co-regulated by microphtalmia transcription factor. J Biol Chem 282:1891–1904. doi:10.1074/jbc.M608572200
Meyerrose TE, Roberts M, Ohlemiller KK, Vogler CA, Wirthlin L, Nolta JA, Sands MS (2008) Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells 26:1713–1722
Michigami T, Kageyama T, Satomura K, Shima M, Yamaoka K, Nakayama M, Ozono K (2002) Novel mutations in the a3 subunit of vacuolar H(+)-adenosine triphosphatase in a Japanese patient with infantile malignant osteopetrosis. Bone 30:436–439. doi:10.1016/S8756-3282(01)00684-6
Nicholls BM, Bredius RG, Hamdy NA, Gerritsen EJ, Lankester AC, Hogendoorn PC, Nesbitt SA, Horton MA, Flanagan AM (2005) Limited rescue of osteoclast-poor osteopetrosis after successful engraftment by cord blood from an unrelated donor. J Bone Miner Res 20:2264–2270. doi:10.1359/JBMR.050807
Pangrazio A, Poliani PL, Megarbane A, Lefranc G, Lanino E, Di Rocco M, Rucci F, Lucchini F, Ravanini M, Facchetti F, Abinum M, Vezzoni P, Villa A, Frattini A (2006) Mutations in OSTM1 (grey lethal) define a particularly severe form of autosomal recessive osteopetrosis with neural involvement. J Bone Miner Res 21:1098–1105. doi:10.1359/jbmr.060403
Quarello P, Forni M, Barberis L, Defilippi C, Campagnoli MF, Silvestro L, Frattini A, Chalhoub N, Vacher J, Ramenghi U (2004) Severe malignant osteopetrosis caused by a GL gene mutation. J Bone Miner Res 19:1194–1199. doi:10.1359/JBMR.040407
Ramirez A, Faupel J, Goebel I, Stiller A, Beyer S, Stockle C, Hasan C, Bode U, Kornak U, Kubisch C (2004) Identification of a novel mutation in the coding region of the grey-lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum Mutat 23:471–476. doi:10.1002/humu.20028
Schulz AS, Classen CF, Mihatsch WA, Sigl-Kraetzig M, Wiesneth M, Debatin KM, Friedrich W, Müller SM (2002) HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood 99:3458–3460. doi:10.1182/blood.V99.9.3458
Scimeca JC, Quincey D, Parrinello H, Romatet D, Grosgeorge J, Gaudray P, Philip N, Fischer A, Carle GF (2003) Novel mutations in the TCIRG1 gene encoding the a3 subunit of the vacuolar proton pump in patients affected by infantile malignant osteopetrosis. Hum Mutat 21:151–157. doi:10.1002/humu.10165
Sobacchi C, Frattini A, Orchard P, Porras O, Tezcan I, Andolina M, Babul-Hirji R, Baric I, Canham N, Chitayat D, Dupuis-Girod S, Ellis I, Etzioni A, Fasth A, Fisher A, Gerritsen B, Gulino V, Horwitz E, Klamroth V, Lanino E, Mirolo M, Musio A, Matthijs G, Nonomaya S, Notarangelo LD, Ochs HD, Superti Furga A, Valiaho J, van Hove JL, Vihinen M, Vujic D, Vezzoni P, Villa A (2001) The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum Mol Genet 10:1767–1773. doi:10.1093/hmg/10.17.1767
Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, Grabowski P, Del Fattore A, Messina C, Errigo G, Coxon FP, Scott DI, Teti A, Rogers MJ, Vezzoni P, Villa A, Helfrich MH (2007) Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39:960–962. doi:10.1038/ng2076
Souraty N, Noun P, Djambas-Khayat C, Chouery E, Pangrazio A, Villa A, Lefranc G, Frattini A, Mégarbané A (2007) Molecular study of six families originating from the Middle-East and presenting with autosomal recessive osteopetrosis. Eur J Med Genet 50:188–199. doi:10.1016/j.ejmg.2007.01.005
Susani L, Pangrazio A, Sobacchi C, Taranta A, Mortier G, Savarirayan R, Villa A, Orchard P, Vezzoni P, Albertini A, Frattini A, Pagani F (2004) TCIRG1-dependent recessive osteopetrosis: mutation analysis, functional identification of the splicing defects and in vitro rescue by U1snRNA. Hum Mutat 24:225–235. doi:10.1002/humu.20076
Tolar J, Bonfim C, Grewal S, Orchard P (2006) Engraftment and survival following hematopoietic stem cell transplantation for osteopetrosis using a reduced intensity conditioning regimen. Bone Marrow Transplant 38:783–787. doi:10.1038/sj.bmt.1705533
Tsuji Y, Ito S, Isoda T, Kajiwara M, Nagasawa M, Morio T, Mizutani S (2005) Successful nonmyeloablative cord blood transplantation for an infant with malignant infantile osteopetrosis. J Pediatr Hematol Oncol 27:495–498. doi:10.1097/01.mph.0000179961.72889.bf
Van Wesenbeeck L, Odgren PR, Coxon FP, Frattini A, Moens P, Perdu B, MacKay CA, Van Hul E, Timmermans JP, Vanhoenacker F, Jacobs R, Peruzzi B, Teti A, Helfrich MH, Rogers MJ, Villa A, Van Hul W (2007) Involvement of PLEKHM1 in osteoclastic vesicular transport and osteopetrosis in incisors absent rats and humans. J Clin Invest 117:919–930. doi:10.1172/JCI30328
Weinberg KI, Kapoor N, Shah AJ, Crooks GM, Kohn DB, Parkman R (2001) Hematopoietic stem cell transplantation for severe combined immune deficiency. Curr Allergy Asthma Rep 1:416–420R. doi:10.1007/s11882-001-0026-2
Xu YQ, Liu ZC (2008) Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev 4:101–112
Acknowledgements
This work was supported by grants from Eurostells (STELLAR) and FIRB/MIUR to P.V. (RBIN04CHXT), from the Fondazione Telethon to C.S. (grant GGP07059), from the Fondazione Cariplo to A.F and from ISS Malattie Rare (New cell therapy approaches for infantile malignant Osteopetrosis) to P.V. The work reported in this paper has also been funded by the N.O.B.E.L. (Network Operativo per la Biomedicina di Eccellenza in Lombardia) Program from Fondazione Cariplo to P.V. and A.V. The technical assistance of Dario Strina and Lucia Susani is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Villa, A., Pangrazio, A., Caldana, E. et al. Prognostic potential of precise molecular diagnosis of Autosomal Recessive Osteopetrosis with respect to the outcome of bone marrow transplantation. Cytotechnology 58, 57–62 (2008). https://doi.org/10.1007/s10616-008-9165-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-008-9165-9