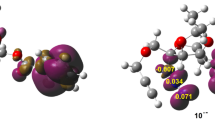
The computational wb97xd/6-311+G(d)(PCM) study confirms without any doubt the polar, stepwise mechanism of hetero-Diels–Alder reactions as illustrated on the example of 5,6-diphenyl-1,2,4-triazine and 2-cyclopropylidene-1,3-dimethylimidazoline. The first reaction stage is the formation of a prereaction molecular complex, which can next convert to two possible zwitterionic intermediates characterized by “cyclic” and “extended” conformation. The first one cyclizes in the second reaction step to the expected six-membered cycloadduct.



Similar content being viewed by others
References
Kiamehr, M.; Khademi, F.; Jafari, B.; Langer, P. Chem. Heterocycl. Compd. 2020, 56, 392.
Merkulova, E. A.; Kolobov, A. V.; Ovchinnikov, K. L.; Khrustalev, V. N.; Nenajdenko, V. G. Chem. Heterocycl. Compd. 2021, 57, 245.
Khramtsova, E. E.; Dmitriev, M. V.; Bormotov, N. I.; Serova, O. A.; Shishkina, L. N.; Maslivets, A. N. Chem. Heterocycl. Compd. 2021, 57, 483.
Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Pure Appl. Chem. 2021, 93, 427.
Lamri, S.; Heddam, A.; Kara, M.; Yahia, W.; Khorief Nacereddine, A. Organics 2021, 2, 57.
Benhamed, L.; Mekelleche, S. M.; Benchouk, W. Organics 2021, 2, 38.
Domingo, L. R.; Sáez, J. A. Org. Biomol. Chem. 2009, 7, 3576.
Jasiński, R. Symmetry 2021, 13, 1911.
Houk, K. N.; Liu, F.; Yang, Z.; Seeman, J. I. Angew. Chem., Int. Ed. 2021, 60, 12660.
Jasiński, R.; Dresler, E. Organics 2020, 1, 49.
Ernd, M.; Heuschmann, M.; Zipse, H. Helv. Chim. Acta 2005, 88, 1491.
Fizer, M.; Slivka, M.; Sidey, V.; Baumer, V.; Fizer, O. J. Mol. Struct. 2021, 1241, 130632.
Fizer, M.; Slivka, M.; Sidey, V.; Baumer, V.; Mariychuk, R. J. Mol. Struct. 2021, 1235, 130227.
Jasiński, R.; Mirosław, B.; Demchuk, O. M.; Babyuk, D.; Łapczuk-Krygier, A. J. Mol. Struct. 2016, 1108, 689.
Kącka-Zych, A. Organics 2020, 1, 36.
Jasiński, R. J. Fluorine Chem. 2018, 206, 1.
Jasiński, R. J. Mol. Graph. Model. 2017, 75, 55.
Jasiński, R. Comput. Theor. Chem. 2014, 1046, 93.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, 2013.
Ríos-Gutiérrez, M.; Domingo, L. R.; Jasiński, R. RSC Adv. 2021, 11, 9698.
Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Organics 2022, 3, 59.
Kącka-Zych, A.; Jasiński, R. New J. Chem. 2021, 45, 9491.
Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110.
Domingo, L. R. RSC Adv. 2014, 4, 32415.
Jasiński, R. Tetrahedron Lett. 2015, 56, 532.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(4/5), 260–262
Supplementary Information
ESM 1
(PDF 561 kb)
Rights and permissions
About this article
Cite this article
Jasiński, R. Stepwise, zwitterionic course of hetero-Diels–Alder reaction between 1,2,4-triazine molecular systems and 2-cyclopropylidene-1,3-dimethylimidazoline. Chem Heterocycl Comp 58, 260–262 (2022). https://doi.org/10.1007/s10593-022-03081-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-022-03081-y