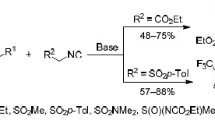
1,3-Dipolar cycloaddition reactions of 3,3,3-trifluoropropene derivatives containing a sulfonyl, sulfamide, or sulfoximine substituent in position 1 with diazomethane proceed with the formation of 3-substituted 4-(trifluoromethyl)-4,5-dihydro-1H-pyrazoles and 3-(trifluoromethyl)-1H-pyrazole, whereas reactions with ethyl diazoacetate and 2,2,2-trifluorodiazoethane lead to the formation of isomeric 5(3)-substituted 4-trifluoromethyl-3,4(4,5)-dihydro-2(1)H-pyrazoles and 4-substituted 5-(trifluoromethyl)-4,5-dihydro-1Hpyrazoles, the stability of which depends on the nature of the heteroatomic substituent. The cycloaddition of 1-sulfonyl- and 1-sulfamoylsubstituted derivatives of 3,3,3-trifluoropropene to C-carbethoxy-N-phenylnitrilimine gives rise to 4-substituted ethyl 1-phenyl-5-(trifluoromethyl)-4,5-dihydro-1H-pyrazole-3-carboxylates and ethyl 1-phenyl-4-(trifluoromethyl)-1H-pyrazole-3-carboxylate.

Similar content being viewed by others
Notes
Hereinafter in the experimental part, the signals of the major isomer are marked with an asterisk (*), the signals of both isomers are marked with two asterisks (**).
References
(a) Faria, J. V.; Vegi, P. F.; Miguita, A. G .C.; dos Santos, M. S.; Boechat, N.; Bernardino, A. M. R. Bioorg. Med. Chem. 2017, 25, 5891. (b) Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F. A. J. Chem. Sci. 2019, 131, 70. (c) Schmidt, A.; Dreger, A. Curr. Org. Chem. 2011, 15, 1423.
(a) Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Gupta, H. Recent Pat. Anti-Infect. Drug Discovery 2009, 4, 154. (b) Turkan, F.; Cetin, A.; Taslimi, P.; Karaman, H. S.; Gulçin, I. Arch. Pharm. 2019, 352, 1800359. (c) Azarifar, D.; Shaebanzadeh, M. Molecules 2002, 7, 885. (d) Malhotra, V.; Pathak, S.; Nath, R.; Mukerjee, D.; Shanker, K. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2002, 41B, 1310. (e) Palaska, E.; Aytemir, M.; Uzbay, T.; Eros, D. Eur. J. Med. Chem. 2001, 36, 639.
(a) Penning, T. D.; Talley, J. J.; Bertenshaw, S. R.; Carter, J. S.; Collins, P. W.; Docter, S.; Graneto, M. J.; Lee, L. F.; Malecha, J. W.; Miyashiro, J. M.; Rogers, R. S.; Rogier, D. J.; Yu, S. S.; Anderson, G. D.; Burton, E. G.; Cogburn, J. N.; Gregory, S. A.; Koboldt, C. M.; Perkins, W. E.; Seibert, K.; Veenhuizen, A. W.; Zhang, Y. Y.; Isakson, P. C. J. Med. Chem. 1997, 40, 1347. (b) Sun, A.; Chandrakumar, N.; Yoon, J.-J.; Plemper, R. K.; Snyder, J. P. Bioorg. Med. Chem. Lett. 2007, 17, 5199. (c) Quan, M. L.; Lam, P. Y. S.; Han, Q.; Pinto, D. J. P.; He, M. Y., Li, R.; Ellis, C. D.; Clark, C. G.; Teleha, C. A.; Sun, J.-H.; Alexander, R. S.; Bai, S.; Luettgen, J. M.; Knabb, R. M.; Wong, P. C.; Wexler, R. R. J. Med. Chem. 2005, 48, 1729. (d) Giornal, F.; Pazenok, S.; Rodefeld, L.; Lui, N.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2013, 152, 2. (e) Foster, R. S.; Jakobi, H.; Harrity, J. P. A. Org. Lett. 2012, 14, 4858. (f) Topchij, M. A.; Zharkova, D. A.; Asachenko, A. F.; Muzalevskiy, V. M.; Chertkov, V. A.; Nenajdenko, V. G.; Nechaev, M. S. Eur. J. Org. Chem. 2018, 27-28, 3750. (g) Muzalevskiy, V. M.; Nenajdenko, V. G. Org. Biomol. Chem. 2018, 16, 7935. (h) Wang, H.; Ning, Y.; Sun, Y.; Sivaguru, P.; Bi, X. Org. Lett. 2020, 22, 2012. (i) Pianoski, K. E.; Poletto, J.; da Silva, M. J. V.; Camargo, J. N. A.; Jacomini, A. P.; Gonçalves, D. S.; Back, D. F.; Moura, S.; Rosa, F. A. Org. Biomol. Chem. 2020, 18, 2524. (j) Zeng, H.; Fang, X.; Yang, Z.; Zhu, C.; Jiang, H. J. Org. Chem. 2021, 86, 2810.
(a) Markitanov, Yu. N.; Timoshenko, V. M.; Shermolovich, Yu. G.; Mykhalchuk, V. L.; Grafova, I. A.; Grafov, A. V. Chem. Heterocycl. Compd. 2016, 52, 503. [Khim. Geterotsikl. Soedin. 2016, 52, 503.] (b) Markitanov, Yu. M.; Timoshenko, V. M.; Shermolovich, Yu. G. Chem. Heterocycl. Compd. 2018, 54, 89. [Khim. Geterotsikl. Soedin. 2018, 54, 89.] (c) Markitanov, Yu. M.; Timoshenko, V. M.; Rudenko, T. V.; Rusanov, E. B.; Shermolovich, Yu. G. J. Sulfur Chem. 2019, 40, 629. b Markitanov, Yu. N.; Timoshenko, V. M.; Rusanov, E. B.; Shermolovich, Yu. G. Chem. Heterocycl. Compd. 2021, 57, 253. [Khim. Geterotsikl. Soedin. 2021, 57, 253.]
(a) Smith, L. I.; Davis, H. R. J. Org. Chem. 1950, 15, 824. (b) Rondestvedt, C. S.; Chang, P. K. J. Am. Chem. Soc. 1955, 77, 6532. (c) Reddy, N.; Balaji, T. S. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 1984, 23B, 983. (d) Kumar, A.; Sharma, A., S.; Malik, N.; Sharma, P.; Kaushik, K.; Saxena, K. K.; Srivastava, V. K. Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem. 2004, 43B, 1532.
Vasin, V. A.; Razin, V. V.; Bezrukova, E. V.; Korovin, D. Yu.; Petrov, P. S.; Somov, N. V. Russ. J. Org. Chem. 2015, 51, 1144. [Zh. Org. Khim. 2015, 51, 1163.]
(a) Hock, K. J.; Mertens, L.; Metze, F. K.; Schmittmann, C.; Koenigs, R. M. Green Chem. 2017, 19, 905. (b) Britton, J.; Jamison, T. F. Angew. Chem., Int. Ed. 2017, 56, 8823. (с) Mertens, M.; Hock, K. J.; Koenigs, R. M. Chem.–Eur. J. 2016, 22, 9542. c Li, F.; Nie, J.; Sun, L.; Zheng, Y.; Ma, J.-A. Angew. Chem., Int. Ed. 2013, 52, 6255. d Li, F.; Wang, J.; Pei, W.; Li, H.; Zhang, H.; Song, M.; Guo, L.; Zhang, A.; Liu, L. Tetrahedron Lett. 2017, 58, 4344. e Mei, H.; Wang, L.; Pajkert R.; Wang, Q.; Xu, J.; Liu, J.; Röschenthaler, G.-V.; Han, J. Org. Lett. 2021, 23, 1130.
Minami, T.; Tokumasu, S.; Mimasu, R.; Hirao, I. Chem. Lett. 1985, 14, 1099.
Gareev, R. D.; Pudovik, A. N. J. Gen. Chem. USSR 1982, 52, 2333. [Zh. Obshch. Khim. 1982, 52, 2637.]
Matoba, K.; Yonemoto, H.; Fukui, M.; Yamazaki, T. Chem. Pharm. Bull. 1984, 32, 3918.
(a) Berestovitskaya, V. M.; Anisimova, N. A.; Gubaidullin, A. T.; Litvinov, I. A.; Berkova, G. A.; Makarova, N. G. Russ. J. Gen. Chem. 2009, 79, 1446. [Zh. Obsh. Khim. 2009, 79, 1090.] (b) Berkova, G. A.; Anisimova, N. A.; Makarova, N. G.; Deiko, L. I.; Berestovitskaya, V. M. Russ. J. Gen. Chem. 2006, 76, 153. [Zh. Obsh. Khim. 2006, 76, 156.]
Plancquaert, M.-A.; Redone, M.; Janousek, Z.; Viehe, H. G. Tetrahedron 1996, 52, 4383.
(а) Yu, H.-B.; Huang, W.-Y. J. Fluorine Chem. 1998, 87, 69. a Gerus, I. I.; Gorbunova M. G.; Vdovenko, S. I.; Yagupol’skii, Yu. L.; Kukhar, V. P. J. Org. Chem. USSR 1990, 26, 1623. [Zh. Org. Khim. 1990, 26, 1877.] (c) Jiang, B.; Xu, Y.-Y.; Yang, J. J. Fluorine Chem. 1994, 67, 83. b Atherton, J. H.; Fields, R. J. Chem. Soc. C 1968, 1507. c Petrella, S.; Aubry, A.; Janvier, G.; Coutant, E. P.; Cartier, A.; Dao, T.-H.; Bonhomme, F. J.; Motreff, L.; Pissis, C.; Bizet, C.; Clermont, D.; Begaud, E.; Retailleau, P.; Munier-Lehmann, H.; Capton, E.; Mayer, C.; Janin, Y. L. Can. J. Chem. 2015, 94, 240.
(a) Muruganantham, R.; Namboothiri, I. N. N. J. Org. Chem. 2010, 75, 2197. (b) Muruganantham, R.; Mobin, S. M.; Namboothiri, I. N. N. Org. Lett. 2007, 9, 1125.
Slobodyanyuk, E. V.; Artamonov, O. S.; Shishkin, O. V.; Mykhailiuk, P. K. Eur. J. Org. Chem. 2014, 12, 2487.
Janin, Y. L. J. Heterocycl. Chem. 2013, 50, 1410.
(a) Kobayashi, Y.; Hamana, H.; Fujino, S.; Ohsawa, A.; Kumadaki, I. J. Org. Chem. 1979, 44, 4930. (b) Gerus, I. I.; Mironetz, R. X.; Kondratov, I. S.; Bezdudny, A. V.; Dmytriv, Y. V.; Shishkin, O. V.; Starova, V. S.; Zaporozhets, O. A.; Tolmachev, A. A.; Mykhailiuk, P. K. J. Org. Chem. 2012, 77, 47.
(a) Elguero, J.; Yranzo, G. I.; Laynez, J.; Jimenez, P.; Menendez, M.; Catalan, J.; De Paz, J. L. G.; Anvia, F.; Taft, R. W. J. Org. Chem. 1991, 56, 3942. (b) Claire, P. P. K.; Coe, P. L.; Jones, C. J.; McCleverly, J. A. J. Fluorine Chem. 1991, 51, 283. (с) Grünebaum, M.; Buchheit, A.; Günther, C.; Wiemhöfer, H.-D. Tetrahedron Lett. 2016, 57, 1555. c Maspero, A.; Giovenzana, G. B.; Monticelli, D.; Tagliapietra, S.; Palmisano, G.; Penoni, A. J. Fluorine Chem. 2012, 139, 53. d Kondrat’ev, P. N.; Skryabina, Z. E.; Saloutin, V. I.; Pashkevich, K. I.; Klyuev, N. A.; Aleksandrov, G. G. Bull. Acad. Sci. USSR., Div. Chem. Sci. 1990, 39, 561. [Izv. Akad. Nauk SSSR, Ser. Khim. 1990, 640.]
(a) Shawali, A. S.; Albar, H. A. Can. J. Chem. 1986, 64, 871. (b) Kulikov, A. S.; Epishina, M. A.; Zhilin, E. S.; Shuvaev, A. D.; Fershtat, L. L.; Makhova, N. N. Mendeleev Commun. 2021, 31, 42.
(a) Zeng, H.; Fang, X.; Yang, Z.; Zhu, C.; Jiang, H. J. Org. Chem. 2021, 86, 2810. (b) Saloutin, V. I.; Skryabina, Z. E.; Kondrat’ev, P. N.; Perevalov, S. G. Russ. J. Org. Chem. 1995, 31, 236. [Zh. Org. Khim. 1995, 31, 266.]
Nickson, T. E. J. Org. Chem. 1988, 53, 3870.
Arndt, F. Org. Synth. 1935, 15, 3.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2021, 57(11), 1107–1115
Supplementary Information
ESM 1
(PDF 2504 kb)
Rights and permissions
About this article
Cite this article
Маrkitanov, Y.N., Тimoshenko, V.М., Мykhaylychenko, S.S. et al. [3+2] Cycloaddition reactions of 1-substituted 3,3,3-trifluoropropenes with diazo compounds and nitrilimines – synthesis of pyrazolines and pyrazoles. Chem Heterocycl Comp 57, 1107–1115 (2021). https://doi.org/10.1007/s10593-021-03029-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-021-03029-8