Abstract
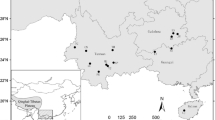
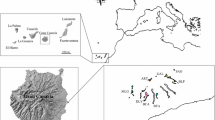
Loss of genetic diversity is expected to be a reason behind the decline of populations of many rare species. To what extent this is true for populations at the range periphery remains to be explored. Alpine species with peripheral lowland populations are an ideal but little-known model system to address this issue. We used 17 microsatellite markers to investigate the genetic diversity and structure of populations of Tofieldia calyculata, a common species in central European mountains, but highly endangered in lowlands. We showed that lowland populations have lower genetic diversity than mountain populations and that the two groups of populations are not clearly differentiated genetically. The species probably survived the last glaciation in refugia in the margins of the Alps and the western Carpathians and some lowland populations likely originated by postglacial colonisation. Some lowland populations may be relictual, but our data did not unequivocally confirm this. Low genetic diversity of lowland populations is likely the result of the reduction of population sizes, limited gene flow, and selfing. Based on data from herbarium specimens from extinct lowland populations, within-population genetic diversity has not changed over the last century suggesting that, under suitable habitat conditions, these populations are able to survive with low levels of genetic diversity. This idea is also supported by the presence of large viable extant populations with very low genetic diversity. Comparisons between modern and historic collection also showed that a large proportion of genetic diversity was lost, due mainly to the extinction of whole populations. Our results provided detailed insight into the recent past of the populations of Tofieldia calyculata, but the genetic diversity of the populations before the twentieth century remains unknown due to the poor quality of old DNA from herbaria samples. Overall, the study indicates that despite reduced genetic diversity, the lowland populations harbour some unique alleles and, with the current levels of genetic diversity, have a chance to survive in the long-term, and thus deserve conservation.






Similar content being viewed by others
References
Bachmann U, Hensen I (2007) Is declining Campanula glomerata threatened by genetic factors? Plant Spec Biol 22:1–10
Beatty GE, Reid N, Provan J (2014) Retrospective genetic monitoring of the threatened yellow marsh saxifrage (Saxifraga hirculus) reveals genetic erosion but provides valuable insights for conservation strategies. Divers Distrib 20:529–537
Beaumont MA (2010) Approximate Bayesian computation in evolution and ecology. Annu Rev Ecol Evol Syst 41:379–406
Bieker VC, Martin MD (2018) Implications and future prospects for evolutionary analyses of DNA in historical herbarium collections. Bot Lett 165:409–418
Brande A (1996) Pollen profile Tegeler See, Brandenburg, Germany. In Supplement to: Brande, A (1996): Type region D-s, Berlin. In: Berglund, BE; Birks, HJB; Ralska-Jasiewiczowa, M; Wright, HE (eds.) Palaeoecological events during the last 15000 years:regional syntheses of palaeoecological studies of lakes and mires in Euro. PANGAEA
Chapuis MP, Estoup A (2007) Microsatellite null alleles and estimation of population differentiation. Mol Biol Evol 24:621–631
Cozzolino S, Cafasso D, Pellegrino G, Musacchio A, Widmer A (2007) Genetic variation in time and space: the use of herbarium specimens to reconstruct patterns of genetic variation in the endangered orchid Anacamptis palustris. Conserv Genet 8:629–639
Dirnböck T, Dullinger S, Grabherr G (2003) A regional impact assessment of climate and land-use change on alpine vegetation. J Biogeogr 30:401–417
Dítě D, Hájek M, Svitková I, Košuthová A, Šoltés R, Kliment J (2018) Glacial-relict symptoms in the western Carpathian flora. Folia Geobot 53:277–300
Dostálek T, Münzbergová Z, Plačková I (2014) High genetic diversity in isolated populations of Thesium ebracteatum at the edge of its distribution range. Conserv Genet 15:75–86
Duwe V, Muller L, Borsch T, Ismail S (2017) Pervasive genetic differentiation among central European populations of the threatened Arnica montana L. and genetic erosion at lower elevations. Perspect Plant Ecol Evol Syst 27:45–56
Duwe V, Muller L, Reichel K, Zippel E, Borsch T, Ismail S (2018) Genetic structure and genetic diversity of the endangered grassland plant Crepis mollis (Jacq.) Asch. as a basis for conservation management in Germany. Conserv Genet 19:527–543
Earl DA, von Holdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Eckert CG, Samis KE, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Mol Ecol 17:1170–1188
Ehrich D (2006) AFLPDAT: a collection of R functions for convenient handling of AFLP data. Mol Ecol Notes 6:603–604
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Frankham R, Ballou J, Briscoe D (2010) Introduction to conservation genetics, 2nd edn. Cambridge University Press, Cambridge
Frey D, Reisch C, Narduzzi-Wicht B, Baur EM, Cornejo C, Alessi M, Schoenenberger N (2017) Historical museum specimens reveal the loss of genetic and morphological diversity due to local extinctions in the endangered water chestnut Trapa natans L. (Lythraceae) from the southern Alpine lake area. Bot J Linn Soc 185:343–358
Gabrielová J, Fialová T, Műnzbergová Z (2011) Critically endangered plant species of the Czech Republic: what is the situation of their endangerment in other European countries? Příroda 31:299–343
Garza JC, Williamson EG (2001) Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10:305–318
Gibson SY, van Der Marel RC, Starzomski BM (2009) Climate change and conservation of leading-edge peripheral populations. Conserv Biol 23:1369–1373
Greimler J, Dobeš C (2000) High genetic diversity and differentiation in relict lowland populations of Gentianella austriaca (A. and J. Kern.) Holub (Gentianaceae). Plant Biol 2:628–637
Hájek M, Horsák M, Tichý L, Hájková P, Dítě D, Jamrichová E (2011) Testing a relict distributional pattern of fen plant and terrestrial snail species at the Holocene scale: a null model approach. J Biogeogr 38:742–755
Hájková P, Grootjans A, Lamentowicz M, Rybníčková E, Madaras M, Opravilová V, Michaelis D, Hájek M, Joosten H, Wolejko L (2012) How a Sphagnum fuscum—dominated bog changed into a calcareous fen: the unique holocene history of a Slovak spring-fed mire. J Quaternary Sci 27:233–243
Hájková P, Jamrichová E, Šolcová A, Frodlová J, Petr L, Dítě D, Hájek M, Horsák M (2020) Can relict-rich communities be of an anthropogenic origin? Palaeoecological insight into conservation strategy for endangered Carpathian travertine fens. Quaternary Sci Rev 234:1–13
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:1
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos T R Soc B 351:1291–1298
Hardie DC, Hutchings JA (2010) Evolutionary ecology at the extremes of species’ ranges. Environ Rev 18:1–20
Hardy OJ, Vekemans X (2002) SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Hendrych R (2003) Poznatky o druhu Ligularia sibirica v Čechách. Preslia 75:39–69
Hensen I, Kilian C, Wagner V, Durka W, Pusch J, Wesche K (2010) Low genetic variability and strong differentiation among isolated populations of the rare steppe grass Stipa capillata L. in central Europe. Plant Biol 12:526–536
Honnay O, Jacquemyn H (2007) Susceptibility of common and rare plant species to the genetic consequences of habitat fragmentation. Conserv Biol 21:823–831
Hunter ML, Hutchinson A (1994) The virtues and shortcomings of parochialism: conserving species that are locally rare, but globally common. Conserv Biol 8:1163–1165
Ilves A, Lanno K, Sammul M, Tali K (2013) Genetic variability, population size and reproduction potential in Ligularia sibirica (L.) populations in Estonia. Conserv Genet 14:661–669
Ivy-ochs S (2015) Glacier variations in the European Alps at the end of the last glaciation. Cuaderm Investig 41:295–315
Janská V, Jimenéz-Alfaro B, Chytrý M, Divíšek J, Anenkhonov O, Korolyuk A, Lashchinskyi N, Culek M (2017) Palaeodistribution modelling of European vegetation types at the last glacial maximum using modern analogues from Siberia: prospects and limitations. Quaternary Sci Rev 159:103–115
Kaplan Z, Danihelka J, Štěpánková J, Bureš P, Zázvorka J, Hroudová Z, Ducháček M, Grulich V, Řepka R, Dančák M, Prančl J, Šumberová K, Wild J, Trávníček B (2015) Distributions of vascular plants in the Czech Republic. Part 1. Preslia 87:417–500
Kirschner J, Kirschnerová L, Bartish I (2011) Conservation status of two isolated populations of Gentiana verna (Gentianaceae) in the Czech Republic: insights from an allozyme analysis. PHYTON-Ann Rei Bot A 51:177–199
Klank C, Ghazoul J, Pluess AR (2012) Genetic variation and plant performance in fragmented populations of globeflowers (Trollius europaeus) within agricultural landscapes. Conserv Genet 13:873–884
Klimešová J, Danihelka J, Chrtek J, de Bello F, Herben T (2017) CLO-PLA: a database of clonal and bud bank traits of central European flora. Ecology 98:1179
Knotek A, Kolář F (2018) Different low-competition island habitats in central Europe harbour similar levels of genetic diversity in relict populations of Galium pusillum agg. (Rubiaceae). Biol J Linn Soc 125:491–507
Kolář F, Fuxová G, Záveská E, Nagano AJ, Hyklová L, Lučanová M, Kudoh H, Marhold K (2016) Northern glacial refugia and altitudinal niche divergence shape genome-wide differentiation in the emerging plant model Arabidopsis arenosa. Mol Ecol 25:3929–3949
Kopeć D, Michalska-Hejduk D (2012) How threatened is the Polish wetland flora? Oceanol Hydrobiol St 41:79–89
Kropf M, Bardy K, Höhn M, Plenk K (2020) Phylogeographical structure and genetic diversity of Adonis vernalis L. (Ranunculaceae) across and beyond the Pannonian region. Flora 262:151497
Lammi A, Siikamäki P, Mustajärvi K (1999) Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conserv Biol 13:1069–1078
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation? J Ecol 94:942–952
Lodhi MA, Ye G, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Mol Biol Rep 12:6–13
Luijten SH, Kéry M, Oostermeijer JGB, den Nijs HJCM (2002) Demographic consequences of inbreeding and outbreeding in Arnica montana: a field experiment. J Ecol 90:593–603
Lutz E, Schneller JJ, Holderegger R (2000) Understanding population history for conservation purposes: population genetics of Saxifraga aizoides (Saxifragaceae) in the lowlands and lower mountains north of the Alps. Am J Bot 87:583–590
Machon N, Guillon JM, Dobigny G, Le Cadre S, Moret J (2001) Genetic variation in the horsetail Equisetum variegatum Schleich., an endangered species in the Parisian region. Biodivers Conserv 10:1543–1554
Mráz P, Gaudeul M, Rioux D, Gielly L, Choler P, Taberlet P (2007) Genetic structure of Hypochaeris uniflora (Asteraceae) suggests vicariance in the Carpathians and rapid post-glacial colonization of the Alps from an eastern Alpine refugium. J Biogeogr 34:2100–2114
Münzbergová Z, Šurinová M, Husáková I, Brabec J (2018) Strong fluctuations in aboveground population size do not limit genetic diversity in populations of an endangered biennial species. Oecologia 187:863–872
Niinemets E, Saarse L, Poska A (2002) Vegetation history and human impact in the Parika area, central Estonia. P Est Acad Sci 51:241–258
Noel F, Machon N, Porcher E (2007) No genetic diversity at molecular markers and strong phenotypic plasticity in populations of Ranunculus nodiflorus, an endangered plant species in France. Ann Bot-London 99:1203–1212
Petr L, Novák J (2014) High vegetation and environmental diversity during the late glacial and early holocene on the example of lowlands in the Czech Republic. Biologia 69:847–862
Pico FX, Mix C, Ouborg NJ, van Groenendael JM (2007) Multigenerational inbreeding in Succisa pratensis: effects on fitness components. Biol Plant 51:185–188
Piry S, Luikart G, Cornuet JM (1999) BOTTLENECK: a program for detecting recent effective population size reductions from allele data frequencies. J Hered 90:502–503
Plenk K, Bardy K, Höhn M, Thiv M, Kropf M (2017) No obvious genetic erosion, but evident relict status at the westernmost range edge of the Pontic-Pannonian steppe plant Linum flavum L.(Linaceae) in central Europe. Ecol Evol 7:6527–6539
Plenk K, Bardy K, Höhn M, Kropf M (2019) Long-term survival and successful conservation? Low genetic diversity but no evidence for reduced reproductive success at the north-westernmost range edge of Poa badensis (Poaceae) in central Europe. Biodivers Conserv 28:1245–1265
Pluess AR, Stöcklin J (2004) Genetic diversity and fitness in Scabiosa columbaria in the Swiss Jura in relation to population size. Conserv Genet 5:145–156
Pokorny P, Sádlo J, Bernardová A (2010) Holocene history of Cladium mariscus (L.) pohl in the Czech republic. Implications for species population dynamics and palaeoecology. Acta Palaeobotanica 50:65–76
Pokorný P, Chytrý M, Juřičková L, Sádlo J, Novák J, Ložek V (2015) Mid-Holocene bottleneck for central European dry grasslands: Did steppe survive the forest optimum in northern Bohemia. Czech Republic? Holocene 25:716–726
Pritchard J, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
R development team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing. https://www.r-project.org
Ralska-Jasiewiczowa M, Rzętkowska A (1987) Pollen and macrofossil stratigraphy of fossil lake sediments at Niechorze I, W. Baltic coast. Acta Paleobotanica 27:153–178
Reisch C, Poschlod P, Wingender R (2002) Genetic variation of Sesleria albicans Kit. ex Schultes (Poaceae): lack of evidence for glacial relict endemism in central Europe. Plant Biol 4:711–719
Reisch C, Poschlod P, Wingender R (2003) Genetic variation of Saxifraga paniculata Mill. (Saxifragaceae): molecular evidence for glacial relict endemism in central Europe. Biol J Linn Soc 80:11–21
Ronikier M, Cieślak E, Korbecka G (2008) High genetic differentiation in the alpine plant Campanula alpina Jacq. (Campanulaceae): evidence for glacial survival in several Carpathian regions and long-term isolation between the Carpathians and the Alps. Mol Ecol 17:1763–1775
Ronikier M, Schneeweiss GM, Schőnswetter P (2012) The extreme disjunction between Beringia and Europe in Ranunculus glacialis s. l. (Ranunculaceae) does not coincide with the deepest genetic split—a story of the importance of temperate mountain ranges in arctic-alpine phylogeography. Mol Ecol 21:5561–5578
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106
Schönswetter P, Stehlík I, Holderegger R, Tribsch A (2005) Molecular evidence for glacial refugia of mountain plants in the European Alps. Mol Ecol 14:3547–3555
Segarra-Moragues JG, Mateu-Andrés I (2007) Levels of allozyme diversity in closely related toadflaxes (Linaria, Plantaginaceae) and their correspondence with the breeding systems of the species. Conserv Genet 8:373–383
Slovák M, Kučera J, Turis P, Zozomová-Lihová J (2012) Multiple glacial refugia and postglacial colonization routes inferred for a woodland geophyte, Cyclamen purpurascens: patterns concordant with the pleistocene history of broadleaved and coniferous tree species. Biol J Linn Soc 105:741–760
Šmarda P (2018) Ploidy level (x). www.pladias.cz
Šmídová A, Münzbergová Z, Plačková I (2011) Genetic diversity of a relict plant species, Ligularia sibirica (L.) Cass. (Asteraceae). Flora 20:151–157
Štěpánková J (ed) (2010) Květena České republiky 8. Academia, Praha
Theodoridis S, Randin C, Szovenyi P, Boucher FC, Patsiou TS, Conti E (2017) How do cold-adapted plants respond to climatic cycles? Interglacial expansion explains current distribution and genomic diversity in Primula farinosa L. Syst Biol 66:715–736
Thiel-Egenter C, Alvarez N, Holderegger R, Tribsch A, Englisch T, Wohlgemuth T, Colli L, Gaudeul M, Gielly L, Linder HP, Negrin R, Niklfeld H, Pellecchia M, Schönswetter P, Taberlet P, Loo M, Winkler M, Gugerli F (2011) Break zones in the distributions of alleles and species in alpine plants. J Biogeogr 38:772–782
Tomimatsu H, Ohara M (2003) Genetic diversity and local population structure of fragmented populations of Trillium camschatcense (Trilliaceae). Biol Conserv 109:249–258
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting gen- otyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vasquéz DLA, Balslev H, Hansen MM, Sklenar P, Romoleroux K (2016) Low genetic variation and high differentiation across sky island populations of Lupinus alopecuroides (Fabaceae) in the northern Andes. Alp Bot 126:135–142
Vlasta T, Jarošová A, Šurinová M, Münzbergová Z (2020) Development of microsatellite markers for the perennial plant Tofieldia calyculata. Appl Plant Sci 8:1–5
Vogler F, Reisch C (2013) Vital survivors: low genetic variation but high germination in glacial relict populations of the typical rock plant Draba aizoides. Biodivers Conserv 22:1301–1316
Wagner V, Durka W, Hensen I (2011) Increased genetic differentiation but no reduced genetic diversity in peripheral vs. central populations of a steppe grass. Am J Bot 98:1173–1179
Windmaißer T, Kattari S, Heubl G, Reisch C (2016) Glacial refugia and postglacial expansion of the alpine–prealpine plant species Polygala chamaebuxus. Ecol Evol 6:7809–7819
Wroblewska A (2008) From the center to the margins of geographical range: molecular history of steppe plant Iris aphylla L. in Europe. Plant Syst and Evol 272:49–65
Acknowledgements
We would like to thank the curators of herbaria in the museums (PRC, PR, CB, HR, LIT, BERN) for providing samples from historical localities in the Czech Republic and samples from the Alps. We also thank: Gabriela Šrámková and Diana L. A. Vásquez for help with data analysis; Andrea Jarošová and Maria Šurinová for advising on the methodology of analysis of herbarium samples; Vojtěch Abraham, Anna Šolcová, Diana L. A. Vásquez and a host of others, including the anonymous reviewers for comments helping us to improve the manuscript. We would like to give thanks to Toomas Kukk for collecting samples from Estonia and to F. Kolář, C. Pachschwöll, T. Pachschwöll and Katja Rembold for collecting samples from the Alps. We would like to thank the many people and organisations involved in gaining permissions and in helping to find suitable populations for research (namely, Norbert Pühringer and Kristina Plenk). The language of the paper has been corrected by Glynn Kirkham.
Funding
This work was supported by the Charles University Grant Agency (Grant No. 1450218).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary conflicts of interests.
Informed consent
We obtained all permission to enter protected areas and for manipulation with the species in countries where the species is protected.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vlasta, T., Münzbergová, Z. Genetic variation in lowland and mountain populations of Tofieldia calyculata and their ability to survive within low levels of genetic diversity. Conserv Genet 23, 605–622 (2022). https://doi.org/10.1007/s10592-022-01439-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-022-01439-5