Abstract
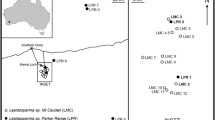
Lepidospartum burgessii is a rare gypsophilic shrub with limited distribution in New Mexico and Texas. Most of the known plants are restricted to two large populations, with a few small, isolated populations scattered in the surrounding area. The low recruitment observed in the two largest populations may be due to low seed set resulting from high inbreeding and/or self-incompatibility. We used eight microsatellite loci to quantify diversity, relatedness, inbreeding, population structure, and frequency of clonal reproduction. Seed collections were made to quantify seed set and germination rates. Overall, there was a moderate level of clonal diversity within patches of L. burgessii indicating asexual growth is important for population persistence. Inbreeding coefficients were high both between and within populations. Most patches showed a significant level of relatedness between individuals. At a fine scale, patches within populations were significantly different from each other, however when all patches were combined, the two populations of L. burgessii were not genetically distinct. Compared to a population of its common congener, Lepidospartum latisquamum, L. burgessii populations had similar measures of diversity, more clonal reproduction, and lower germination rates. High relatedness and inbreeding may explain the low seed set and recruitment in L. burgessii, however factors such as insect herbivory and precipitation changes may further depress recruitment.


Similar content being viewed by others
References
Allen CD (2007) Interactions across spatial scales among forest dieback, fire, and erosion in northern New Mexico landscapes. Ecosystems 10:797–808
Amat ME, Silvertown J, Vargas P (2013) Strong spatial genetic structure reduces reproductive success in the critically endangered plant genus Pseudomisopates. J Her 104:692–703
Arnaud-Haond S, Alberto F, Teixeira S, Procaccini G, Serrao EA, Duarte CM (2005) Assessing genetic diversity in clonal organisms: low diversity or low resolution? Combining power and cost efficiency in selecting markers. J Her 96:434–440
Brennan AC, Harris SA, Hiscock SJ (2013) The population genetics of sporophytic self-incompatibility in three hybridizing Senecio (Asteraceae) species with contrasting population histories. Evolution 67:1347–1367
Breshears DD, Cobb NS, Rich PM, Price KP, Allen CD, Balice RG, Romme WH, Kastens JH, Floyd ML, Belnap J, Anderson JJ, Myers OB, Meyer CW (2005) Regional vegetation die-off in response to global-change-type drought. Proc Natl Acad Sci USA 102:15144–15148
Byers DL, Meagher TR (1997) A comparison of demographic characteristics in a rare and common species of Eupatorium. Ecol Appl 7:519–530
Calflora (2013) Lepidospartum latisquamum S. Watson The Calflora Database. http://www.calflora.org/cgi-bin/species_query.cgi?where-taxon=Lepidospartum+latisquamum. Accessed May 21 2013
Charlesworth D (1985) Distribution of dioecy and self-incompatibility in angiosperms. In: Greenwoog PJ, Harvey PH, Slatkin M (eds) Evolution: essays in honor of John Maynard Smith. Cambridge University Press, Cambridge, pp 237–268
Clark AG, Hubisz MJ, Bustamante CD, Williamson SH, Nielsen R (2005) Ascertainment bias in studies of human genome-wide polymorphism. Genome Res 15:1496–1502
R Core Team (2014) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Coulon A, Fitzpatrick JW, Bowman R, Stith BM, Makarewich CA, Stenzler LM, Lovette IJ (2008) Congruent population structure inferred from dispersal behaviour and intensive genetic surveys of the threatened Florida scrub-jay (Aphelocoma cœrulescens). Mol Ecol 17:1685–1701
Davis J, Childers D, Kuhn D (1999) Clonal variation in a Florida Bay Thalassia testudinum meadow: molecular genetic assessment of population structure. Mar Ecol Prog Ser 186:127–136
De Mauro MM (1993) Relationship of breeding system to rarity in the lakeside daisy (Hymenoxys acaulis var. glabra). Conserv Biol 7:542–550
Dorken ME, Eckert CG (2001) Severely reduced sexual reproduction in northern populations of a clonal plant, Decodonverticillatus (Lythraceae). J Ecol 89:339–350
Duminil J, Hardy OJ, Petit RJ (2009) Plant traits correlated with generation time directly affect inbreeding depression and mating system and indirectly genetic structure. BMC Evol Biol 9:177
Earl DA, vonHoldt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361
Eriksson O (1989) Seedling dynamics and life histories in clonal plants. Oikos 55:231–238
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Fant JB, Holmstrom RM, Sirkin E, Etterson JR, Masi S (2008) Genetic structure of threatened native populations and propagules used for restoration in a clonal species, American Beachgrass (Ammophila breviligulata Fern.). Restor Ecol 16:594–603
Fant JB, Kramer A, Sirkin E, Havens K (2013) Genetics of reintroduced populations of the narrowly endemic thistle, Cirsium pitcheri (Asteraceae). Botany 91:301–308
Fant JB, Havens K, Keller JM, Radosavljevic A, Yates ED (2014) The influence of contemporary and historic landscape features on the genetic structure of the sand dune endemic, Cirsium pitcheri (Asteraceae). Heredity 112:519–530
Fernández-Mazuecos M, Jiménez-Mejías P, Rotllan-Puig X, Vargas P (2014) Narrow endemics to Mediterranean islands: moderate genetic diversity but narrow climatic niche of the ancient, critically endangered Naufraga (Apiaceae). Perspect Plant Ecol Evol Syst 16:190–202
Fiedler PL (1987) Life history and population dynamics of rare and common Mariposa Lilies (Calochortus Pursh: liliaceae). J Ecol 75:977–995
Frankham R, Ballou JD, Eldridge MDB, Lacy RC, Ralls K, Dudash MR, Fenster CB (2011) Predicting the probability of outbreeding depression. Conserv Biol 25:465–475
Galpern P, Manseau M, Hettinga P, Smith K, Wilson P (2012) Allelematch: an R package for identifying unique multilocus genotypes where genotyping error and missing data may be present. Mol Ecol Resour 12:771–778
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Goudet J (2005) hierfstat, a package for r to compute and test hierarchical F-statistics. Mol Ecol Notes 5:184–186
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond B 351:1291–1298
Hannan GL, Orick MW (2000) Isozyme diversity in Iris cristata and the threatened glacial endemic I. Lacustris (Iridaceae). Am J Bot 87:293–301
Hao YQ, Zhao XF, She DY, Xu B, Zhang DY, Liao WJ (2012) The role of late-acting self-incompatibility and early-acting inbreeding depression in governing female fertility in monkshood. Aconitum kusnezoffii. PLoS One 7:e47034
Jiménez-Mejías P, Fernández-Mazuecos M, Amat ME, Vargas P (2015) Narrow endemics in European mountains: high genetic diversity within the monospecific genus Pseudomisopates (Plantaginaceae) despite isolation since the late Pleistocene. J Biogeogr 42:1455–1468
Kim E, Zaya D, Fant J, Ashley M (2014) Genetic factors accelerate demographic decline in rare Asclepias species. Conserv Genet 16(2):1–11
Ladyman JAR, Gegick P (2000) The status of Lepidospartum burgessii (Burgess Broomshrub or Gypsum Broomscale). In: Maschinski J, Holter L (eds) Southwestern rare and endangered plants: Proceedings of the Third Conference. U.S. Department of Agriculture, Fort Collins, pp 116–127
Ladyman JAR, Delay L, Gegick P, Bogan M (1999) Status and reproductive biology of Lepidospartum burgessii (Burgess broomshrub or gypsum broomscale). (ed. Program NH), p. 110. NM Heritage Program, University of NM and USDI Geological Survey at University of NM
Lesica P, Yurkewycz R, Crone EE (2006) Rare plants are common where you find them. Am J Bot 93:454–459
Liu G, Hegarty MJ, Edwards KJ, Hiscock SJ, Abbott RJ (2004) Isolation and characterization of microsatellite loci in Senecio. Mol Ecol Notes 4:611–614
Lynch M, Ritland K (1999) Estimation of pairwise relatedness with molecular markers. Genetics 152:1753–1766
Moore MJ, Jansen RK (2007) Origins and biogeography of gypsophily in the Chihuahuan Desert plant group Tiquilia Subg. Eddya (Boraginaceae). Syst Bot 32:392–414
Muirhead CA, Lande R (1997) Inbreeding depression under joint selfing, outcrossing, and asexuality. Evolution 51:1409–1415
Nei M (1972) Genetic distance between populations. Am Nat 106:000–283
Peakall R, Smouse PE (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Peakall R, Smouse P (2012) GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28(19):2537–2539
Pelser PB, Kennedy AH, Tepe EJ, Shidler JB, Nordenstam B, Kadereit JW, Watson LE (2010) Patterns and causes of incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. Am J Bot 97:856–873
Prati D, Schmid B (2000) Genetic differentiation of life-history traits within populations of the clonal plant Ranunculus reptans. Oikos 90:442–456
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rousset F (2008) genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Schuelke M (2000) An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol 18:233–234
Travis SE, Proffitt CE, Ritland K (2004) Population structure and inbreeding vary with successional stage in created Spartina alterniflora marshes. Ecol Appl 14:1189–1202
Turner BL (1977) Lepidospartum burgessii (Asteraceae, Senecioneae), a remarkable new gypsophilic species from Trans-Pecos Texas. Wrightia 5:354–355
USDA (2013) Lepidospartum latisquamum S. Watson: Nevada broomsage. http://plants.usda.gov/java/profile?symbol=LELA4. Accessed May 21 2013
Vallejo-Marin M, Dorken ME, Barrett SCH (2010) The ecological and evolutionary consequences of clonality for plant mating. Annu Rev Ecol Evol Syst 41:193–213
Valtueña FJ, Rodriguez-Riano T, Espinosa F, Ortega-Olivencia A (2010) Self-sterility in two Cytisus species (Leguminosae, Papilionoideae) due to early-acting inbreeding depression. Am J Bot 97:123–135
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Wagenius S, Lonsdorf E, Neuhauser C (2007) Patch aging and the S-allee effect: breeding system effects on the demographic response of plants to habitat fragmentation. Am Nat 169:383–397
Wagenius S, Hangelbroek HH, Ridley CE, Shaw RG (2010) Biparental inbreeding and interremnant mating in a perennial prairie plant: fitness consequences for progeny in their first eight years. Evolution 64:761–771
Widen B, Cronberg N, Widen M (1994) Genotypic diversity, molecular markers and spatial distribution of genets in clonal plants, a literature survey. Folia Geobotan 29:245–263
Acknowledgments
The authors wish to acknowledge the National Fish and Wildlife Foundation, the Mohamed Bin Zayed Conservation Fund, and the BLM Plant Conservation Program for funding. We thank Guadalupe National Park, the Conservation Land Management internship program and its participants for field work, John Keller for laboratory work, and Dean Tonnena for collections. Comments by anonymous reviewers greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Williams, E.W., Cheung, R., Siegel, C. et al. Persistence of the gypsophile Lepidospartum burgessii (Asteraceae) through clonal growth and limited gene flow. Conserv Genet 17, 1201–1211 (2016). https://doi.org/10.1007/s10592-016-0855-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10592-016-0855-0