Abstract
Submicroscopic inversions have contributed significantly to the genomic divergence between humans and chimpanzees over evolutionary time. Those microinversions which are flanked by segmental duplications (SDs) are presumed to have originated via non-allelic homologous recombination between SDs arranged in inverted orientation. However, the nature of the mechanisms underlying those inversions which are not flanked by SDs remains unclear. We have investigated 35 such inversions, ranging in size from 51-nt to 22056-nt, with the goal of characterizing the DNA sequences in the breakpoint-flanking regions. Using the macaque genome as an outgroup, we determined the lineage specificity of these inversions and noted that the majority (N = 31; 89%) were associated with deletions (of length beween 1-nt and 6754-nt) immediately adjacent to one or both inversion breakpoints. Overrepresentations of both direct and inverted repeats, ≥ 6-nt in length and capable of non-B DNA structure formation, were noted in the vicinity of breakpoint junctions suggesting that these repeats could have contributed to double strand breakage. Inverted repeats capable of cruciform structure formation were also found to be a common feature of the inversion breakpoint-flanking regions, consistent with these inversions having originated through the resolution of Holliday junction-like cruciforms. Sequences capable of non-B DNA structure formation have previously been implicated in promoting gross deletions and translocations causing human genetic disease. We conclude that non-B DNA forming sequences may also have promoted the occurrence of mutations in an evolutionary context, giving rise to at least some of the inversion/deletions which now serve to distinguish the human and chimpanzee genomes.



Similar content being viewed by others
Abbreviations
- aCGH:
-
array-based comparative genomic hybridization
- bp:
-
base pairs
- DSBs:
-
double strand breaks
- DR:
-
direct repeat
- HJ:
-
Holliday junction
- IR:
-
inverted repeat
- LINEs:
-
long interspersed nuclear elements
- LTR:
-
long terminal repeat
- MIR:
-
mammalian-wide interspersed repeat
- nt:
-
nucleotides
- SD:
-
segmental duplication
- SE:
-
symmetric element
- SINEs:
-
small interspersed nuclear elements
- SSA-type:
-
single-strand annealing-type
References
Abeysinghe SS, Chuzhanova N, Krawczak M, Ball EV, Cooper DN (2003) Translocation and gross deletion breakpoints in human inherited disease and cancer I: Nucleotide composition and recombination-associated motifs. Hum Mutat 22:229–244
Bacolla A, Wells RD (2004) Non-B DNA conformations, genomic rearrangements, and genetic diseases. J Biol Chem 279:47411–47414
Bacolla A, Wojciechowska M, Kosmider B, Larson JE, Wells RD (2006) The involvement of non-B DNA structures in gross chromosomal rearrangements. DNA Repair 5:1161–1170
Bacolla A, Larson JE, Collins JR, Li J, Milosavljevic A, Stenson PD, Cooper DN, Wells RD (2008) Abundance and length of simple repeats in vertebrate genomes are determined by their structural properties. Genome Res 10:1545–1553
Bacolla A, Jaworski A, Larson JE, Jakupciak JP, Chuzhanova N, Abeysinghe SS, O'Connell CD, Cooper DN, Wells RD (2004) Breakpoints of gross deletions coincide with non-B DNA conformations. Proc Natl Acad Sci USA 101:14162–14167
Ball EV, Stenson PD, Krawczak M, Cooper DN, Chuzhanova NA (2005) Micro-deletions and micro-insertions causing human genetic disease: common mechanisms of mutagenesis and the role of local DNA sequence complexity. Hum Mutat 26:205–213
Birmingham EC, Lee SA, McCulloch RD, Baker MD (2004) Testing predictions of the double-strand break repair model relating to crossing over in mammalian cells. Genetics 168:1539–1555
Chaisson MJ, Raphael BJ, Pevzner PA (2006) Microinversions in mammalian evolution. Proc Natl Acad Sci USA 103:19824–19829
Cullen M, Perfetto SP, Klitz W, Nelson G, Carrington M (2002) High-resolution patterns of meiotic recombination across the human major histocompatibility complex. Am J Hum Genet 71:759–776
Déclais AC, Lilley DM (2008) New insight into the recognition of branched DNA structure by junction-resolving enzymes. Curr Opin Struct Biol 18:86–95
Feuk L, MacDonald JR, Tang T, Carson AR, Li M, Rao G, Khaja R, Scherer SW (2005) Discovery of human inversion polymorphisms by comparative analysis of human and chimpanzee DNA sequence assemblies. PLoS Genet 1:e56
Gibbs RA, Rogers J, Katze MG et al (2007) Evolutionary and biomedical insights from the rhesus macaque genome. Science 316:222–234
Gusev VD, Nemytikova LA, Chuzhanova NA (1999) On the complexity measures of genetic sequences. Bioinformatics 15:994–999
Han K, Lee J, Meyer TJ, Wang J, Sen SK, Srikanta D, Liang P, Batzer MA (2007) Alu recombination-mediated structural deletions in the chimpanzee genome. PLoS Genet 3:1939–1949
Inagaki H, Ohye T, Kogo H, Kato T, Bolor H, Taniguchi M, Shaikh TH, Emanuel BS, Kurahashi H (2009) Chromosomal instability mediated by non-B DNA: cruciform conformation and not DNA sequence is responsible for recurrent translocation in humans. Genome Res 19:191–198
Ip SC, Rass U, Blanco MG, Flynn HR, Skehel JM, West SC (2008) Identification of Holliday junction resolvases from humans and yeast. Nature 456:357–361
Kehrer-Sawatzki H, Cooper DN (2008) Molecular mechanisms of chromosomal rearrangement during primate evolution. Chrom Res 16:41–56
Khuu PA, Voth AR, Hays FA, Ho PS (2006) The stacked-X DNA Holliday junction and protein recognition. J Mol Recognit 19:234–242
Kurahashi H, Inagaki H, Ohye T, Kogo H, Kato T, Emanuel BS (2006) Chromosomal translocations mediated by palindromic DNA. Cell Cycle 5:1297–1303
Lee J, Han K, Meyer TJ, Kim HS, Batzer MA (2008) Chromosomal inversions between human and chimpanzee lineages caused by retrotransposons. PLoS ONE 3:e4047
Losch FO, Bredenbeck A, Hollstein VM, Walden P, Wrede P (2007) Evidence for a large double-cruciform DNA structure on the X chromosome of human and chimpanzee. Hum Genet 122:337–343
Ma J, Zhang L, Suh BB, Raney BJ, Burhans RC, Kent WJ, Blanchette M, Haussler D, Miller W (2006) Reconstructing contiguous regions of an ancestral genome. Genome Res 16:1557–1565
Marino-Ramirez L, Spouge JL, Kanga GC, Landsman D (2004) Statistical analysis of over-represented words in human promoter sequences. Nucleic Acids Res 32:949–958
Mikkelsen TS, Hillier LW, Eichler EE et al (2005) Initial sequence of the chimpanzee genome and comparison with the human genome. Nature 437:69–87
Murayama Y, Kurokawa Y, Mayanagi K, Iwasaki H (2008) Formation and branch migration of Holliday junctions mediated by eukaryotic recombinases. Nature 451:1018–1021
Myers S, Bottolo L, Freeman C, McVean G, Donnelly P (2005) A fine-scale map of recombination rates and hotspots across the human genome. Science 310:321–324
Myers S, Spencer CCA, Auton A, Bottolo L, Freeman C, Donnelly P, McVean G (2006) The distribution and causes of meiotic recombination in human genome. Biochem Soc Trans 34:526–530
Napierala M, Bacolla A, Wells RD (2005) Increased negative superhelical density in vivo enhances the genetic instability of triplet repeat sequences. J Biol Chem 280:37366–37376
Raghavan SC, Lieber MR (2006) DNA structures at chromosomal translocation sites. Bioessays 28:480–494
Rozen S, Skaletsky H, Marszalek JD, Minx PJ, Cordum HS, Waterston RH, Wilson RK, Page DC (2003) Abundant gene conversion between arms of palindromes in human and ape Y chromosomes. Nature 423:873–876
Szamalek JM, Goidts V, Chuzhanova N, Hameister H, Cooper DN, Kehrer-Sawatzki H (2005) Molecular characterisation of the pericentric inversion that distinguishes human chromosome 5 from the homologous chimpanzee chromosome. Hum Genet 117:168–176
Szamalek JM, Cooper DN, Schempp W, Minich P, Kohn M, Hoegel J, Goidts V, Hameister H, Kehrer-Sawatzki H (2006) Polymorphic micro-inversions contribute to the genomic variability of humans and chimpanzees. Hum Genet 119:103–112
Wang G, Vasquez KM (2004) Naturally occurring H-DNA-forming sequences are mutagenic in mammalian cells. Proc Natl Acad Sci USA 101:13448–13453
Wang G, Vasquez KM (2006) Non-B DNA structure-induced genetic instability. Mutation Res 598:103–119
Wang G, Christensen LA, Vasquez KM (2006) Z-DNA-forming sequences generate large-scale deletions in mammalian cells. Proc Natl Acad Sci USA 103:2677–2682
Wells RD (2007) Non-B DNA conformations, mutagenesis, and diseases. Trends Biochem Sci 32:271–278
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Fengtang Yang.
Electronic supplementary material
Below is the link to the electronic supplementary material
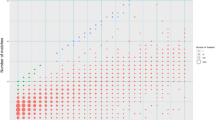
Fig. S1
Alignment dot-plots. The panels depict the alignments of the 35 inversions between the human genomic plus strands and the chimpanzee genomic minus strands, including ~10 nt of non-inverted DNA sequences at both ends of each sequence. The genomic coordinates for the human sequences are given in Table 1. The genomic coordinates for the orthologous chimpanzee sequences following BLAT searches were as follows: panel 1, chr10:12136276-12136329; panel 2, chr10:116844843-116844954; panel 3, chr10:53922309-53922470; panel 4, chr10:126100936-126101213; panel 5, chr7:95515505-95515791; panel 6, chr11:113304150-113304491; panel 7, chr10:11718879-11719507; panel 8, chr6:72091037-72091659; panel 9, chr5:35330988-35331976; panel 10, chr16:85430135-85431243; panel 11, chr6:12644332-12645386; panel 12, chr7:137167855-137169253; panel 13, chr5:20180206-20181756; panel 14, chr5:119533003-119534741; panel 15, chr11:87128690-87129600; panel 16, chr7:124887989-124892425; panel 17, chr10:36549197-36554419; panel 18, chr5:136839424-136840615; panel 19, chr5:147021847-147035189; panel 20, chr4:43620932-43622318; panel 21, chr2a:8128093-8132020; panel 22, chr13:82801968-82807302; panel 23, chr4:44508164-44511072; panel 24, chr2b:160340903-160341245; panel 25, chr8:136861733-136862113; panel 26, chr11:131738359-131760399; panel 27, chr2b:180789656-180789856; panel 28, chr4:98469220-98469364; panel 29, chr13:115695710-115696114; panel 30, chr14:48396349-48396784; panel 31, chr17:73655852-73656289; panel 32, chr18:18030460-18030973; panel 33, chr13:113714126-113714652; panel 34, chr14:94557537-94558775; panel 35, chr6:168834882-168837248 (PDF 626 kb)
Fig. S2
Localization of the sequences included in the datasets (D) used to determine the repeat element frequency in regions flanking the breakpoints of the inversions >1000-nt. Vertical black lines indicate the positions of the breakpoints. Dataset 1 (D1) included 500-nt from the non-inverted region whereas dataset 2 (D2) included 500-nt directly flanking the inversion breakpoints within the inverted segments. Dataset (D3) included the sequences within the deleted regions in the ancestral sequence. The frequency of SINE and LINE elements in these datasets was investigated in both human and chimpanzee (PPT 119 kb)
Fig. S3
Case 32 comprises a 594 bp inversion in the human genome (bold and ^). In the chimpanzee genome, it is characterized by the presence of a 146 bp IR in which the left hand repeat copy is located almost entirely outside of the proximal breakpoint (with the exception of the last 8 bp, broken black arrows), whereas the right hand repeat copy is located mostly (118/146) within the inverted region. In human, the entire left arm of the IR has been lost along with an additional 71 bp (lower case), whereas a 14 bp sequence (blue) has been inserted de novo just distal to the inversion breakpoint. The 14 nt is exactly complementary to the proximally inverted region (red arrows). We speculate that the 146 bp IRs mediated the inversion through the formation of a cruciform structure and that following deletion of the left hand repeat copy, a novel cruciform structure was created during the resolution of the secondary structure intermediate. In case 35, a human-specific inversion of 2352 bp occurred (bold and ^). The inversion occurred with stark precision within the last bases of a 669 bp IR (black arrows), with no gain or loss of nts with respect to the ancestral chimpanzee genomic sequence. Sequence alignment analyses support the conclusion that the IR sequences became inverted between the two species. The inversion is readily explicable by the parsimony model presented in Fig. 3, whereby the basal HJ was resolved through the non-intersecting strands whereas the apical HJ was resolved through the intersecting strands. Case 4 represents an inversion of 258 bp in human (bold and ^). In addition, a 330 bp segment flanked by a TGA microhomology (yellow highlighting) has been deleted from the chimpanzee genome downstream of the distal inversion breakpoint (lower case). By contrast, a 173 bp insertion (blue) is evident at the proximal breakpoint, representing an inverted copy of the last 173 bp of the inverted segment (broken red arrows). This inversion is explicable in terms of the model presented in Fig. 3, Structures V and VI. In case 23, the human-specific inversion involves 2885 nt in human and 2889 nt in chimpanzee (bold and ^). However, because part of the inversion is present as an inverted repeat in human, the precise position of the upstream breakpoint is ambiguous. Thus, the inversion could have occurred over a 2252 nt interval in human and 2225 nt in the chimpanzee (left broken red arrow). Interestingly, the distal breakpoint, which is unambiguous, occurs over a repeating (AC)5 tract, a sequence known to be capable of adopting a left-handed Z-DNA conformation under conditions of torsional stress (negative supercoiling). In the human derivative chromosome, an inverted duplication of 634 bp has occurred, thereby creating a large IR (broken red arrows). In addition, a 193 bp segment has been transposed from downstream of the inversion breakpoint to upstream of the proximal inversion breakpoint (grey highlighting), whereas its last 12 nt have become duplicated distally to the inversion (blue arrows). [The transposition is independent of the inversion size]. Most (10 nt) of the Z-DNA-forming tract was deleted in the process (lower case). We conclude that the large IR was likely to have been critical both to the inversion process and to the direct transposition event. Finally, we consider case 5 in which a chimpanzee-specific inversion of 267 bp occurred (bold and ^). In the ancestral human genome, the inversion breakpoints occurred at a homologous GA dinucleotide (yellow highlighting). In addition, Mfold analyses predicted several folded branched hairpin structures with moderate stability at both ends (red, black and green arrows). Upon inversion in the chimpanzee genome, a larger hairpin structure was predicted at the proximal breakpoint (red arrows). We postulate that these short IRs played a crucial role in this inversion, as outlined in Fig. 3 for the simpler cases (DOC 41.5 kb)
Fig. S4
Mfold modeling of the inversion #34 cruciform structure. The two 178-nt inverted repeat sequences flanking the breakpoints of human inversion #34 (GTTAG---ATATTTA and TAAATAT---CTAATA, Fig. 2) were joined by a 4-nt loop (TTTT) and used as the query. The Figure illustrates the only result returned by the analysis (DOC 55.5 kb)
Table S1
Sequences of PCR primers used to amplify across the inversion breakpoints in the species in which the inversion occurred (DOC 49 kb)
Table S2
Coordinates of the inversions investigated by dotplot analysis and genes affected by these inversions (XLS 41.5 kb)
Table S3
Number of SINE/LINE elements in the human genome sequence flanking the 24 human-specific inversion breakpoints (DOC 48 kb)
Table S4
Number of SINE/LINE elements in the chimpanzee genome sequence flanking the sites of the 24 human-specific inversion breakpoints (DOC 47.5 kb)
Table S5
Number of SINE/LINE elements in the chimpanzee genome sequence flanking the 11 chimpanzee-specific inversion breakpoints (DOC 34 kb)
Table S6
Number of SINE/LINE elements in the human genome sequence flanking the sites of the 11 chimpanzee-specific inversion breakpoints (DOC 34 kb)
Table S7
Total numbers of SINE or LINE elements in the human and chimpanzee genomes according to Gibbs et al. (2007). (DOC 28.5 kb)
Table S8
Motifs associated with site-specific cleavage/recombination, high frequency mutation and gene rearrangement or recombination ≥ 5 bp of length (DOC 66.5 kb)
Table S9
SINE/LINE frequency in the breakpoint-flanking regions of the 24 human-specific inversions in human as compared to chimpanzee (DOC 59 kb)
Table S10
SINE/LINE frequencies in the breakpoint-flanking regions of the 11 chimpanzee-specific inversions in human as compared to chimpanzee (DOC 44.5 kb)
Rights and permissions
About this article
Cite this article
Kolb, J., Chuzhanova, N.A., Högel, J. et al. Cruciform-forming inverted repeats appear to have mediated many of the microinversions that distinguish the human and chimpanzee genomes. Chromosome Res 17, 469–483 (2009). https://doi.org/10.1007/s10577-009-9039-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10577-009-9039-9