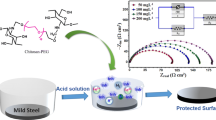
Abstract
Natural biopolymer chitosan organic compound (COC) has been used as a copper corrosion inhibitor in molar hydrochloric medium. This study was conducted by weight loss, polarization curves and electrochemical impedance spectroscopy measurements. Scanning electron microscopy, energy dispersive X-ray spectrometry and atomic force microscopy studies were used to characterize the surface of uninhibited and inhibited copper specimens. The study of the temperature effect was carried out to reveal the chemical nature of adsorption. The inhibition efficiency tends to increase by increasing inhibitor concentration to reach a maximum of 87% at 10−1 mg L−1. The values of inhibitor efficiency estimated by different electrochemical and gravimetric methods indicate the performance of copper in HCl medium containing COC. Adsorption of COC was found to follow the Langmuir adsorption isotherm. In order to get a better understanding of the relationship between the inhibition efficiency and molecular structure of COC, quantum chemical and molecular dynamics simulation approaches were performed to get a better understanding of the relationship between the inhibition efficiency and molecular structure of chitosan.




















Similar content being viewed by others
References
Alsabagh AM, Elsabee MZ, Moustafa YM, Elfky A, Morsi RE (2014) Corrosion inhibition efficiency of some hydrophobically modified chitosan surfactants in relation to their surface active properties. Egypt J Pet 23:349–359
Antonijević MM, Milić SM, Šerbula SM, Bogdanović GD (2005) The influence of chloride ions and benzotriazole on the corrosion behavior of Cu37Zn brass in alkaline medium. Electrochim Acta 50:3693–3701
Antonijević MM, Milić SM, Petrović MB (2009) Films formed on copper surface in chloride media in the presence of azoles. Corros Sci 51:1228–1237
Arukalam IO, Madufor IC, Ogbobe O, Oguzie EE (2015) Inhibition of mild steel corrosion in sulfuric acid medium by hydroxyethyl cellulose. Chem Eng Commun 202:112–122
Ashassi-Sorkhabi H, Seifzadeh D, Hosseini MG (2008) EN, EIS and polarization studies to evaluate the inhibition effect of 3H-phenothiazin-3-one, 7-dimethylamin on mild steel corrosion in 1M HCl solution. Corros Sci 50:3363–3370
Awad MK, Mustafa MR, Elnga MMA (2010) Computational simulation of the molecular structure of some triazoles as inhibitors for the corrosion of metal surface. J Mol Struct (Thoechem) 959:66–74
Awad MK, Metwally MS, Soliman SA, El-Zomrawy AA, Bedair MA (2014) Experimental and quantum chemical studies of the effect of poly ethylene glycol as corrosion inhibitors of aluminum surface. J Ind Eng Chem 20:796–808
Barouni K, Bazzi L, Salghi R, Mihit M, Hammouti B, Albourine A, El Issami S (2008) Some amino acids as corrosion inhibitors for copper in nitric acid solution. Mater Lett 62:3325–3327
Bousskri A, Anejjar A, Messali M, Salghi R, Benali O, Karzazi Y, Jodeh S, Zougagh M, Ebenso EE, Hammouti B (2015) Corrosion inhibition of carbon steel in aggressive acidic media with 1-(2-(4-chlorophenyl)-2-oxoethyl)pyridazinium bromide. J Mol Liq 211:1000–1008
Cheng S, Chen S, Liu T, Chang X, Yin Y (2007) Carboxymenthylchitosan as an ecofriendly inhibitor for mild steel in 1M HCl. Mater Lett 61:3276–3280
Deyab MA, Essehli R, El Bali B (2015) Inhibition of copper corrosion in cooling seawater under flowing conditions by novel pyrophosphate. RSC Adv 5:64326–64334
El Adnani Z, McHarfi M, Sfaira M, Benzakour M, Benjelloun AT, Ebn M (2013) Touhami, DFT theoretical study of 7-R-3methylquinoxalin-2(1H)-thiones (RH; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Corros Sci 68:223–230
El Ibrahimi B, Soumoue A, Jmiai A, Bourzi H, Oukhrib R, El Mouaden K, El Issami S, Bazzi L (2016) Computational study of some triazole derivatives (un- and protonated forms) and their copper complexes in corrosion inhibition process. J Mol Struct 1125:93–102
El-Haddad MN (2013) Chitosan as a green inhibitor for copper corrosion in acidic medium. Int J Biol Macromol 55:142–149
El-Haddad MN (2014) Hydroxyethylcellulose used as an eco-friendly inhibitor for 1018 c-steel corrosion in 3.5% NaCl solution. Carbohydr Polym 112:595–602
Fekry AM, Mohamed RR (2010) Acetyl thiourea chitosan as an eco-friendly inhibitor for mild steel in sulphuric acid medium. Electrochim Acta 55:1933–1939
Finšgar M, Milošev I (2010) Inhibition of copper corrosion by 1,2,3-benzotriazole: a review. Corros Sci 52:2737–2749
Frenkel D, Smit B (2002) Chapter 3—Monte Carlo simulations, understanding molecular simulation, 2nd edn. Academic Press, San Diego, pp 23–61
Frisch AMJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, JEP Jr., Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ, Gaussian, Inc., Wallingford CT, 2009
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1874
Gomma GK (1998) Effect of azole compounds on corrosion of copper in acid medium. Mater Chem Phys 56:27–34
Habbache N, Alane N, Djerad S, Tifouti L (2009) Leaching of copper oxide with different acid solutions. Chem Eng J 152:503–508
Hassan HH, Abdelghani E, Amin MA (2007) Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives: part I. Polariz EIS Stud Electrochim Acta 52:6359–6366
Herreros O, Quiroz R, Viñals J (1999) Dissolution kinetics of copper, white metal and natural chalcocite in Cl2/Cl–media. Hydrometallurgy 51:345–357
Hu K, Zhuang J, Zheng C, Ma Z, Yan L, Gu H, Zeng X, Ding J (2016) Effect of novel cytosine-l-alanine derivative based corrosion inhibitor on steel surface in acidic solution. J Mol Liq 222:109–117
Ismail KM (2007) Evaluation of cysteine as environmentally friendly corrosion inhibitor for copper in neutral and acidic chloride solutions. Electrochim Acta 52:7811–7819
Khaled KF (2010) Corrosion control of copper in nitric acid solutions using some amino acids—a combined experimental and theoretical study. Corros Sci 52:3225–3234
Khaled KF, Hackerman N (2004) Ortho-substituted anilines to inhibit copper corrosion in aerated 0.5M hydrochloric acid. Electrochim Acta 49:485–495
Khaled KF, Fadl-Allah SA, Hammouti B (2009) Some benzotriazole derivatives as corrosion inhibitors for copper in acidic medium: experimental and quantum chemical molecular dynamics approach. Mater Chem Phys 117:148–155
Khan PF, Shanthi V, Babu RK, Muralidharan S, Barik RC (2015) Effect of benzotriazole on corrosion inhibition of copper under flow conditions. J Environ Chem Eng 3:10–19
Kim E-Y, Kim M-S, Lee J-C, Jeong J, Pandey BD (2011) Leaching kinetics of copper from waste printed circuit boards by electro-generated chlorine in HCl solution. Hydrometallurgy 107:124–132
Kirkpatrick S, Gelatt CD, Vecchi MP (1983) Optimization by simulated annealing. Science 220:671–680
Kovačević N, Kokalj A (2011a) Analysis of molecular electronic structure of imidazole- and benzimidazole-based inhibitors: a simple recipe for qualitative estimation of chemical hardness. Corros Sci 53:909–921
Kovačević N, Kokalj A (2011b) DFT study of interaction of azoles with Cu(111) and Al(111) surfaces: role of azole nitrogen atoms and dipole–dipole interactions. J Phys Chem C 115:24189–24197
Larabi L, Benali O, Mekelleche SM, Harek Y (2006) 2-Mercapto-1-methylimidazole as corrosion inhibitor for copper in hydrochloric acid. Appl Surf Sci 253:1371–1378
Lebrini M, Lagrenée M, Vezin H, Gengembre L, Bentiss F (2005) Electrochemical and quantum chemical studies of new thiadiazole derivatives adsorption on mild steel in normal hydrochloric acid medium. Corros Sci 47:485–505
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Li X, Deng S, Fu H (2009) Synergistic inhibition effect of red tetrazolium and uracil on the corrosion of cold rolled steel in H3PO4 solution: weight loss, electrochemical, and AFM approaches. Mater Chem Phys 115:815–824
Liao QQ, Yue ZW, Yang D, Wang ZH, Li ZH, Ge HH, Li YJ (2011) Self-assembled monolayer of ammonium pyrrolidine dithiocarbamate on copper detected using electrochemical methods, surface enhanced Raman scattering and quantum chemistry calculations. Thin Solid Films 519:6492–6498
Liu Y, Zou C, Yan X, Xiao R, Wang T, Li M (2015) β-Cyclodextrin modified natural chitosan as a green inhibitor for carbon steel in acid solutions. Ind Eng Chem Res 54:5664–5672
Menaka R, Subhashini S (2016) Chitosan Schiff base as eco-friendly inhibitor for mild steel corrosion in 1M HCl. J Adhes Sci Technol 30:1622–1640
Mourya P, Banerjee S, Singh MM (2014) Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros Sci 85:352–363
Mu G, Li X (2005) Inhibition of cold rolled steel corrosion by Tween-20 in sulfuric acid: weight loss, electrochemical and AFM approaches. J Colloid Interface Sci 289:184–192
Núñez L, Reguera E, Corvo F, González E, Vazquez C (2005) Corrosion of copper in seawater and its aerosols in a tropical island. Corros Sci 47:461–484
Oguzie EE, Li Y, Wang FH (2007) Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J Colloid Interface Sci 310:90–98
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Rhazi M, Desbrières J, Tolaimate A, Rinaudo M, Vottero P, Alagui A, El Meray M (2002) Influence of the nature of the metal ions on the complexation with chitosan.: application to the treatment of liquid waste. Eur Polym J 38:1523–1530
Rocca E, Bertrand G, Rapin C, Labrune JC (2001) Inhibition of copper aqueous corrosion by non-toxic linear sodium heptanoate: mechanism and ECAFM study. J Electroanal Chem 503:133–140
Sangeetha Y, Meenakshi S, SairamSundaram C (2015) Corrosion mitigation of N-(2-hydroxy-3-trimethyl ammonium)propyl chitosan chloride as inhibitor on mild steel. Int J Biol Macromol 72:1244–1249
Sangeetha Y, Meenakshi S, Sairam Sundaram C (2016) Investigation of corrosion inhibitory effect of hydroxyl propyl alginate on mild steel in acidic media. J Appl Polym Sci 133 n/a-n/a
Sangeetha Y, Meenakshi S, Sairam Sundaram C (2016b) Corrosion inhibition of aminated hydroxyl ethyl cellulose on mild steel in acidic condition. Carbohydr Polym 150:13–20
Satapathy AK, Gunasekaran G, Sahoo SC, Amit K, Rodrigues PV (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51:2848–2856
Sherif EM, Park S-M (2006a) Inhibition of copper corrosion in acidic pickling solutions by N-phenyl-1,4-phenylenediamine. Electrochim Acta 51:4665–4673
Sherif EM, Park S-M (2006b) Effects of 2-amino-5-ethylthio-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in aerated acidic pickling solutions. Electrochim Acta 51:6556–6562
Sherif EM, Park S-M (2006c) 2-Amino-5-ethyl-1,3,4-thiadiazole as a corrosion inhibitor for copper in 3.0% NaCl solutions. Corros Sci 48:4065–4079
Sherif E-SM, Erasmus RM, Comins JD (2007) Effects of 3-amino-1,2,4-triazole on the inhibition of copper corrosion in acidic chloride solutions. J Colloid Interface Sci 311:144–151
Sherif E-SM, Erasmus RM, Comins JD (2008) Inhibition of copper corrosion in acidic chloride pickling solutions by 5-(3-aminophenyl)-tetrazole as a corrosion inhibitor. Corros Sci 50:3439–3445
Shukla SK, Quraishi MA, Prakash R (2008) A self-doped conducting polymer “polyanthranilic acid”: an efficient corrosion inhibitor for mild steel in acidic solution. Corros Sci 50:2867–2872
Shukla SK, Singh AK, Ahamad I, Quraishi MA (2009) Streptomycin: a commercially available drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Mater Lett 63:819–822
Simonović AT, Petrović MB, Radovanović MB, Milić SM, Antonijević MM (2014) Inhibition of copper corrosion in acidic sulphate media by eco-friendly amino acid compound. Chem Pap 68:362–371
Subramanian R, Lakshminarayanan V (2002) Effect of adsorption of some azoles on copper passivation in alkaline medium. Corros Sci 44:535–554
Suzuki S, Shibutani N, Mimura K, Isshiki M, Waseda Y (2006) Improvement in strength and electrical conductivity of Cu–Ni–Si alloys by aging and cold rolling. J Alloys Compd 417:116–120
Tan YS, Srinivasan MP, Pehkonen SO, Chooi SYM (2006) Effects of ring substituents on the protective properties of self-assembled benzenethiols on copper. Corros Sci 48:840–862
Tawfik SM (2015) Alginate surfactant derivatives as an ecofriendly corrosion inhibitor for carbon steel in acidic environments. RSC Adv 5:104535–104550
Tromans D, SiIva JC (1996) Anodic behavior of copper in iodide solutions. J Electrochem Soc 143:458–465
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polym 140:314–341
Vathsala K, Venkatesha TV, Praveen BM, Nayana KO (2010) Electrochemical generation of Zn-chitosan composite coating on mild steel and its corrosion studies. Engineering 2:580–584
Vera R, Bastidas F, Villarroel M, Oliva A, Molinari A, Ramírez D, del Río R (2008) Corrosion inhibition of copper in chloride media by 1,5-bis(4-dithiocarboxylate-1-dodecyl-5-hydroxy-3-methylpyrazolyl)pentane. Corros Sci 50:729–736
Wang B, Du M, Zhang J, Gao CJ (2011) Electrochemical and surface analysis studies on corrosion inhibition of Q235 steel by imidazoline derivative against CO2 corrosion. Corros Sci 53:353–361
Wang H-X, Zhang Y, Cheng J-L, Li Y-S (2015) High temperature oxidation resistance and microstructure change of aluminized coating on copper substrate. Trans Nonferrous Met Soc China 25:184–190
Wazzan NA (2015) DFT calculations of thiosemicarbazide, arylisothiocynates, and 1-aryl-2,5-dithiohydrazodicarbonamides as corrosion inhibitors of copper in an aqueous chloride solution. J Ind Eng Chem 26:291–308
Yadav M, Kumar S, Sinha RR, Bahadur I, Ebenso EE (2015a) New pyrimidine derivatives as efficient organic inhibitors on mild steel corrosion in acidic medium: electrochemical, SEM, EDX, AFM and DFT studies. J Mol Liq 211:135–145
Yadav M, Sinha RR, Sarkar TK, Bahadur I, Ebenso EE (2015b) Application of new isonicotinamides as a corrosion inhibitor on mild steel in acidic medium: electrochemical, SEM, EDX, AFM and DFT investigations. J Mol Liq 212:686–698
Yilmaz N, Fitoz A, Ergun Ü, Emregül KC (2016) A combined electrochemical and theoretical study into the effect of 2-((thiazole-2-ylimino)methyl)phenol as a corrosion inhibitor for mild steel in a highly acidic environment. Corros Sci 111:110–120
Zhang D-Q, Gao L-X, Zhou G-D (2005) Inhibition of copper corrosion in aerated hydrochloric acid solution by amino-acid compounds. J Appl Electrochem 35:1081–1085
Zhang D-Q, Cai Q-R, He X-M, Gao L-X, Zhou G-D (2008a) Inhibition effect of some amino acids on copper corrosion in HCl solution. Mater Chem Phys 112:353–358
Zhang D-Q, Cai Q-R, Gao L-X, Lee KY (2008b) Effect of serine, threonine and glutamic acid on the corrosion of copper in aerated hydrochloric acid solution. Corros Sci 50:3615–3621
Zhang P, Zhu Q, Su Q, Guo B, Cheng S-K (2016) Corrosion behavior of T2 copper in 3.5% sodium chloride solution treated by rotating electromagnetic field. Trans Nonferrous Met Soc China 26:1439–1446
Acknowledgments
Laboratory of Engineering and Materials Science (LISM), University of Reims Champagne Ardenne, France is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jmiai, A., El Ibrahimi, B., Tara, A. et al. Chitosan as an eco-friendly inhibitor for copper corrosion in acidic medium: protocol and characterization. Cellulose 24, 3843–3867 (2017). https://doi.org/10.1007/s10570-017-1381-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-017-1381-z