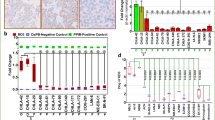
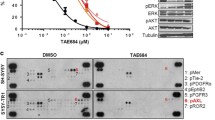
Abstract
Neuroblastoma (NB) progression is branded with hematogenous metastasis and frequent relapses. Despite intensive multimodal clinical therapy, outcomes for patients with progressive disease remain poor, with negligible long-term survival. Therefore, understanding the acquired molecular rearrangements in NB cells with therapy pressure and developing improved therapeutic strategies is a critical need to improve the outcomes for high-risk NB patients. We investigated the rearrangement of MMP9 in NB with therapy pressure, and unveiled the signaling that facilitates NB evolution. Radiation-treatment (RT) significantly increased MMP9 expression/activity, and the induced enzyme activity was persistently maintained across NB cell lines. Furthermore, RT-triggered NFκB transcriptional activity and this RT-induced NFκB were required/adequate for MMP9 maintenance. RT-triggered NFκB-dependent MMP9 actuated a second-signaling feedback to NFκB, facilitating a NFκB-MMP9-NFκB positive feedback cycle (PFC). Critically, MMP9-NFκB feedback is mediated by MMP9-dependent activation of IKKβ and ERK phosphotransferase activity. Beyond its tumor invasion/metastasis function, PFC-dependent MMP9 lessens RT-induced apoptosis and favors survival pathway through the activation of NFκB signaling. In addition, PFC-dependent MMP9 regulates 19 critical molecular determinants that play a pivotal role in tumor evolution. Interestingly, seven of 19 genes possess NFκB-binding sites, demonstrating that MMP9 regulates these molecules by activating NFκB. Collectively, these data suggest that RT-triggered NFκB-dependent MMP9 actuates feedback to NFκB though IKKβ- and ERK1/2-dependent IκBα phosphorylation. This RT-triggered PFC prompts MMP9-dependent survival advantage, tumor growth, and dissemination. Targeting therapy-pressure-driven PFC and/or selective inhibition of MMP9 maintenance could serve as promising therapeutic strategies for treatment of progressive NB.










Similar content being viewed by others
References
Ala M, Mohammad Jafari R, Ala M, Agbele AT, Hejazi SM, Tavangar SM, et al. Sumatriptan alleviates radiation-induced oral mucositis in rats by inhibition of NF-kB and ERK activation, prevention of TNF-alpha and ROS release. Arch Oral Biol. 2020;119: 104919. https://doi.org/10.1016/j.archoralbio.2020.104919.
Ara T, Fukuzawa M, Kusafuka T, Komoto Y, Oue T, Inoue M, et al. Immunohistochemical expression of MMP-2, MMP-9, and TIMP-2 in neuroblastoma: association with tumor progression and clinical outcome. J Pediatr Surg. 1998;33(8):1272–8. https://doi.org/10.1016/s0022-3468(98)90167-1.
Aravindan N, Madhusoodhanan R, Ahmad S, Johnson D, Herman TS. Curcumin inhibits NFkappaB mediated radioprotection and modulate apoptosis related genes in human neuroblastoma cells. Cancer Biol Ther. 2008a;7(4):569–76. https://doi.org/10.4161/cbt.7.4.5534.
Aravindan N, Madhusoodhanan R, Natarajan M, Herman TS. Alteration of apoptotic signaling molecules as a function of time after radiation in human neuroblastoma cells. Mol Cell Biochem. 2008b;310(1–2):167–79. https://doi.org/10.1007/s11010-007-9678-0.
Aravindan N, Veeraraghavan J, Madhusoodhanan R, Herman TS, Natarajan M. Curcumin regulates low-linear energy transfer gamma-radiation-induced NFkappaB-dependent telomerase activity in human neuroblastoma cells. Int J Radiat Oncol Biol Phys. 2011;79(4):1206–15. https://doi.org/10.1016/j.ijrobp.2010.10.058.
Aravindan S, Natarajan M, Awasthi V, Herman TS, Aravindan N. Novel synthetic monoketone transmute radiation-triggered NFkappaB-dependent TNFalpha cross-signaling feedback maintained NFkappaB and favors neuroblastoma regression. PLoS ONE. 2013a;8(8): e72464. https://doi.org/10.1371/journal.pone.0072464.
Aravindan S, Natarajan M, Herman TS, Aravindan N. Radiation-induced TNFalpha cross signaling-dependent nuclear import of NFkappaB favors metastasis in neuroblastoma. Clin Exp Metas. 2013b;30(6):807–17. https://doi.org/10.1007/s10585-013-9580-y.
Aravindan S, Natarajan M, Herman TS, Awasthi V, Aravindan N. Molecular basis of “hypoxic” breast cancer cell radio-sensitization: phytochemicals converge on radiation induced Rel signaling. Radiat Oncol. 2013c;8:46. https://doi.org/10.1186/1748-717X-8-46.
Aravindan N, Aravindan S, Pandian V, Khan FH, Ramraj SK, Natt P, et al. Acquired tumor cell radiation resistance at the treatment site is mediated through radiation-orchestrated intercellular communication. Int J Radiat Oncol Biol Phys. 2014;88(3):677–85. https://doi.org/10.1016/j.ijrobp.2013.11.215.
Aravindan S, Ramraj S, Kandasamy K, Thirugnanasambandan SS, Somasundaram DB, Herman TS, et al. Hormophysa triquerta polyphenol, an elixir that deters CXCR4- and COX2-dependent dissemination destiny of treatment-resistant pancreatic cancer cells. Oncotarget. 2017;8(4):5717–34. https://doi.org/10.18632/oncotarget.13900.
Ardi VC, Van den Steen PE, Opdenakker G, Schweighofer B, Deryugina EI, Quigley JP. Neutrophil MMP-9 proenzyme, unencumbered by TIMP-1, undergoes efficient activation in vivo and catalytically induces angiogenesis via a basic fibroblast growth factor (FGF-2)/FGFR-2 pathway. J Biol Chem. 2009;284(38):25854–66. https://doi.org/10.1074/jbc.M109.033472.
Asuthkar S, Velpula KK, Nalla AK, Gogineni VR, Gondi CS, Rao JS. Irradiation-induced angiogenesis is associated with an MMP-9-miR-494-syndecan-1 regulatory loop in medulloblastoma cells. Oncogene. 2014;33(15):1922–33. https://doi.org/10.1038/onc.2013.151.
Backstrom JR, Lim GP, Cullen MJ, Tokes ZA. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40). J Neurosci. 1996;16(24):7910–9.
Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825(1):29–36. https://doi.org/10.1016/j.bbcan.2011.10.001.
Beliveau A, Mott JD, Lo A, Chen EI, Koller AA, Yaswen P, et al. Raf-induced MMP9 disrupts tissue architecture of human breast cells in three-dimensional culture and is necessary for tumor growth in vivo. Genes Dev. 2010;24(24):2800–11. https://doi.org/10.1101/gad.1990410.
Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15(8–9):519–26. https://doi.org/10.1016/s0945-053x(97)90026-3.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44. https://doi.org/10.1038/35036374.
Bhoopathi P, Chetty C, Kunigal S, Vanamala SK, Rao JS, Lakka SS. Blockade of tumor growth due to matrix metalloproteinase-9 inhibition is mediated by sequential activation of beta1-integrin, ERK, and NF-kappaB. J Biol Chem. 2008;283(3):1545–52. https://doi.org/10.1074/jbc.M707931200.
Chavali PL, Saini RK, Zhai Q, Vizlin-Hodzic D, Venkatabalasubramanian S, Hayashi A, et al. TLX activates MMP-2, promotes self-renewal of tumor spheres in neuroblastoma and correlates with poor patient survival. Cell Death Dis. 2014;5: e1502. https://doi.org/10.1038/cddis.2014.449.
Chen B, Liu J, Ho TT, Ding X, Mo YY. ERK-mediated NF-kappaB activation through ASIC1 in response to acidosis. Oncogenesis. 2016;5(12): e279. https://doi.org/10.1038/oncsis.2016.81.
Cheng Y, Dong Q, Sun LR, Yang CM, Jiang BX. Correlation between expression of MMP-2, MMP-9, TIMP-2, TIMP-1 and metastasis of neuroblastoma. Zhonghua Zhong Liu Za Zhi. 2005;27(3):164–6.
Chou CH, Teng CM, Tzen KY, Chang YC, Chen JH, Cheng JC. MMP-9 from sublethally irradiated tumor promotes Lewis lung carcinoma cell invasiveness and pulmonary metastasis. Oncogene. 2012;31(4):458–68. https://doi.org/10.1038/onc.2011.240.
Deryugina EI, Quigley JP. Pleiotropic roles of matrix metalloproteinases in tumor angiogenesis: contrasting, overlapping and compensatory functions. Biochim Biophys Acta. 2010;1803(1):103–20. https://doi.org/10.1016/j.bbamcr.2009.09.017.
Dwir D, Giangreco B, Xin L, Tenenbaum L, Cabungcal JH, Steullet P, et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol Psychiatry. 2020;25(11):2889–904. https://doi.org/10.1038/s41380-019-0393-5.
Dwivedi A, Slater SC, George SJ. MMP-9 and -12 cause N-cadherin shedding and thereby beta-catenin signalling and vascular smooth muscle cell proliferation. Cardiovasc Res. 2009;81(1):178–86. https://doi.org/10.1093/cvr/cvn278.
Ethell IM, Ethell DW. Matrix metalloproteinases in brain development and remodeling: synaptic functions and targets. J Neurosci Res. 2007;85(13):2813–23. https://doi.org/10.1002/jnr.21273.
Farabegoli F, Govoni M, Spisni E, Papi A. Epigallocatechin-3-gallate and 6-OH-11-O-hydroxyphenanthrene limit BE(2)-C neuroblastoma cell growth and neurosphere formation in vitro. Nutrients. 2018;10(9). https://doi.org/10.3390/nu10091141.
Farina AR, Mackay AR. Gelatinase B/MMP-9 in tumour pathogenesis and progression. Cancers (basel). 2014;6(1):240–96. https://doi.org/10.3390/cancers6010240.
Farina AR, Tacconelli A, Vacca A, Maroder M, Gulino A, Mackay AR. Transcriptional up-regulation of matrix metalloproteinase-9 expression during spontaneous epithelial to neuroblast phenotype conversion by SK-N-SH neuroblastoma cells, involved in enhanced invasivity, depends upon GT-box and nuclear factor kappaB elements. Cell Growth Differ. 1999;10(5):353–67.
Fiore E, Fusco C, Romero P, Stamenkovic I. Matrix metalloproteinase 9 (MMP-9/gelatinase B) proteolytically cleaves ICAM-1 and participates in tumor cell resistance to natural killer cell-mediated cytotoxicity. Oncogene. 2002;21(34):5213–23. https://doi.org/10.1038/sj.onc.1205684.
Fowlkes JL, Enghild JJ, Suzuki K, Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269(41):25742–6.
Gingis-Velitski S, Loven D, Benayoun L, Munster M, Bril R, Voloshin T, et al. Host response to short-term, single-agent chemotherapy induces matrix metalloproteinase-9 expression and accelerates metastasis in mice. Cancer Res. 2011;71(22):6986–96. https://doi.org/10.1158/0008-5472.CAN-11-0629.
Gogineni VR, Kargiotis O, Klopfenstein JD, Gujrati M, Dinh DH, Rao JS. RNAi-mediated downregulation of radiation-induced MMP-9 leads to apoptosis via activation of ERK and Akt in IOMM-Lee cells. Int J Oncol. 2009;34(1):209–18.
Hou H, Zhang G, Wang H, Gong H, Wang C, Zhang X. High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction. Neural Regen Res. 2014;9(11):1154–62. https://doi.org/10.4103/1673-5374.135318.
Hsu CC, Huang SF, Wang JS, Chu WK, Nien JE, Chen WS, et al. Interplay of N-Cadherin and matrix metalloproteinase 9 enhances human nasopharyngeal carcinoma cell invasion. BMC Cancer. 2016;16(1):800. https://doi.org/10.1186/s12885-016-2846-4.
Huang H. Matrix Metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel). 2018;18(10). https://doi.org/10.3390/s18103249.
J W. Mesencyhme-epithelial interactions in gastrointestinal development and tumorigenesis [PhD] 2011.
Jadhav U, Mohanam S. Response of neuroblastoma cells to ionizing radiation: modulation of in vitro invasiveness and angiogenesis of human microvascular endothelial cells. Int J Oncol. 2006;29(6):1525–31.
Kalavska K, Cierna Z, Karaba M, Minarik G, Benca J, Sedlackova T, et al. Prognostic role of matrix metalloproteinase 9 in early breast cancer. Oncol Lett. 2021;21(2):78. https://doi.org/10.3892/ol.2020.12339.
Kataoka H, Uchino H, Iwamura T, Seiki M, Nabeshima K, Koono M. Enhanced tumor growth and invasiveness in vivo by a carboxyl-terminal fragment of alpha1-proteinase inhibitor generated by matrix metalloproteinases: a possible modulatory role in natural killer cytotoxicity. Am J Pathol. 1999;154(2):457–68. https://doi.org/10.1016/s0002-9440(10)65292-3.
Khan FH, Pandian V, Ramraj SK, Aravindan S, Natarajan M, Azadi S, et al. RD3 loss dictates high-risk aggressive neuroblastoma and poor clinical outcomes. Oncotarget. 2015;6(34):36522–34. https://doi.org/10.18632/oncotarget.5204.
Kim YH, Kwon HJ, Kim DS. Matrix metalloproteinase 9 (MMP-9)-dependent processing of betaig-h3 protein regulates cell migration, invasion, and adhesion. J Biol Chem. 2012;287(46):38957–69. https://doi.org/10.1074/jbc.M112.357863.
Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41(2):271–90. https://doi.org/10.1007/s00726-010-0689-x.
Kunigal S, Lakka SS, Joseph P, Estes N, Rao JS. Matrix metalloproteinase-9 inhibition down-regulates radiation-induced nuclear factor-kappa B activity leading to apoptosis in breast tumors. Clin Cancer Res. 2008;14(11):3617–26. https://doi.org/10.1158/1078-0432.CCR-07-2060.
Kuyvenhoven JP, Molenaar IQ, Verspaget HW, Veldman MG, Palareti G, Legnani C et al. Plasma MMP-2 and MMP-9 and their inhibitors TIMP-1 and TIMP-2 during human orthotopic liver transplantation. The effect of aprotinin and the relation to ischemia/reperfusion injury. Thromb Haemost. 2004;91(3):506–13. https://doi.org/10.1160/TH03-05-0272.
Lauricella-Lefebvre MA, Castronovo V, Sato H, Seiki M, French DL, Merville MP. Stimulation of the 92-kD type IV collagenase promoter and enzyme expression in human melanoma cells. Invasion Metastasis. 1993;13(6):289–300.
Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci U S A. 1996;93(14):7069–74. https://doi.org/10.1073/pnas.93.14.7069.
Li XY, Huang GH, Liu QK, Yang XT, Wang K, Luo WZ, et al. Porf-2 inhibits tumor cell migration through the MMP-2/9 signaling pathway in neuroblastoma and glioma. Front Oncol. 2020;10:975. https://doi.org/10.3389/fonc.2020.00975.
London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: a report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29(24):3286–92. https://doi.org/10.1200/JCO.2010.34.3392.
Lu Y, Liu B, Liu Y, Yu X, Cheng G. Dual effects of active ERK in cancer: a potential target for enhancing radiosensitivity. Oncol Lett. 2020;20(2):993–1000. https://doi.org/10.3892/ol.2020.11684.
Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8(11):437–41. https://doi.org/10.1016/s0962-8924(98)01362-2.
Madhusoodhanan R, Natarajan M, Veeraraghavan J, Herman TS, Jamgade A, Singh N, et al. NFkappaB signaling related molecular alterations in human neuroblastoma cells after fractionated irradiation. J Radiat Res. 2009;50(4):311–24. https://doi.org/10.1269/jrr.08110.
Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. Neuroblastoma Nat Rev Dis Primers. 2016;2:16078. https://doi.org/10.1038/nrdp.2016.78.
Misko A, Ferguson T, Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem. 2002;83(4):885–94. https://doi.org/10.1046/j.1471-4159.2002.01200.x.
Mohan N, N A, TS H. Nitric oxide-mediated inhibition of NFkB regulates hyperthermia-induced apoptosis. . 49th Annual meeting of the Radiation Research Society and 20th Annual meeting of the North American Hyperthermia Society. 2002:abstract.
Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–73. https://doi.org/10.1016/j.cardiores.2005.12.002.
Nyormoi O, Mills L, Bar-Eli M. An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ. 2003;10(5):558–69. https://doi.org/10.1038/sj.cdd.4401209.
Ortega N, Behonick DJ, Colnot C, Cooper DN, Werb Z. Galectin-3 is a downstream regulator of matrix metalloproteinase-9 function during endochondral bone formation. Mol Biol Cell. 2005;16(6):3028–39. https://doi.org/10.1091/mbc.e04-12-1119.
Ozdemir E, Kakehi Y, Okuno H, Yoshida O. Role of matrix metalloproteinase-9 in the basement membrane destruction of superficial urothelial carcinomas. J Urol. 1999;161(4):1359–63.
Perng DW, Chang KT, Su KC, Wu YC, Chen CS, Hsu WH, et al. Matrix metalloprotease-9 induces transforming growth factor-beta(1) production in airway epithelium via activation of epidermal growth factor receptors. Life Sci. 2011;89(5–6):204–12. https://doi.org/10.1016/j.lfs.2011.06.008.
Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biology: Journal of the International Society for Matrix Biology. 2007;26(8):587–96. https://doi.org/10.1016/j.matbio.2007.07.001.
Reinhard SM, Razak K, Ethell IM. A delicate balance: role of MMP-9 in brain development and pathophysiology of neurodevelopmental disorders. Front Cell Neurosci. 2015;9:280. https://doi.org/10.3389/fncel.2015.00280.
Ribatti D, Alessandri G, Vacca A, Iurlaro M, Ponzoni M. Human neuroblastoma cells produce extracellular matrix-degrading enzymes, induce endothelial cell proliferation and are angiogenic in vivo. Int J Cancer. 1998;77(3):449–54. https://doi.org/10.1002/(sici)1097-0215(19980729)77:3%3c449::aid-ijc22%3e3.0.co;2-1.
Roderfeld M, Graf J, Giese B, Salguero-Palacios R, Tschuschner A, Muller-Newen G, et al. Latent MMP-9 is bound to TIMP-1 before secretion. Biol Chem. 2007;388(11):1227–34. https://doi.org/10.1515/BC.2007.123.
Roeb E, Schleinkofer K, Kernebeck T, Potsch S, Jansen B, Behrmann I, et al. The matrix metalloproteinase 9 (mmp-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J Biol Chem. 2002;277(52):50326–32. https://doi.org/10.1074/jbc.M207446200.
Sans-Fons MG, Sole S, Sanfeliu C, Planas AM. Matrix metalloproteinase-9 and cell division in neuroblastoma cells and bone marrow macrophages. Am J Pathol. 2010;177(6):2870–85. https://doi.org/10.2353/ajpath.2010.090050.
Santana VM, Furman WL, McGregor LM, Billups CA. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112(12):2796–801. https://doi.org/10.1002/cncr.23507.
Sato H, Seiki M. Regulatory mechanism of 92 kDa type IV collagenase gene expression which is associated with invasiveness of tumor cells. Oncogene. 1993;8(2):395–405.
Shin Y, Yoon SH, Choe EY, Cho SH, Woo CH, Rho JY, et al. PMA-induced up-regulation of MMP-9 is regulated by a PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp Mol Med. 2007;39(1):97–105. https://doi.org/10.1038/emm.2007.11.
Soleyman-Jahi S, Sadeghi F, Afshari Z, Barati T, Ghasemi S, Muhammadnejad S, et al. Anti-neoplastic effects of aprotinin on human breast cancer cell lines: In vitro study. Adv Clin Exp Med. 2019;28(2):151–7. https://doi.org/10.17219/acem/89770.
Solt LA, May MJ. The IkappaB kinase complex: master regulator of NF-kappaB signaling. Immunol Res. 2008;42(1–3):3–18. https://doi.org/10.1007/s12026-008-8025-1.
Somasundaram DB, Subramanian K, Aravindan S, Yu Z, Natarajan M, Herman T, et al. De novo regulation of RD3 synthesis in residual neuroblastoma cells after intensive multi-modal clinical therapy harmonizes disease evolution. Sci Rep. 2019;9(1):11766. https://doi.org/10.1038/s41598-019-48034-2.
Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200(4):448–64. https://doi.org/10.1002/path.1400.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. https://doi.org/10.1146/annurev.cellbio.17.1.463.
Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumor promoter. Oncogene. 2000;19(8):1102–13. https://doi.org/10.1038/sj.onc.1203347.
Stetler-Stevenson WG. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990;9(4):289–303.
Sugiura Y, Shimada H, Seeger RC, Laug WE, DeClerck YA. Matrix metalloproteinases-2 and -9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer Res. 1998;58(10):2209–16.
Suzuki M, Raab G, Moses MA, Fernandez CA, Klagsbrun M. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J Biol Chem. 1997;272(50):31730–7. https://doi.org/10.1074/jbc.272.50.31730.
Thomasset N, Lochter A, Sympson CJ, Lund LR, Williams DR, Behrendtsen O, et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am J Pathol. 1998;153(2):457–67. https://doi.org/10.1016/S0002-9440(10)65589-7.
Tlsty TD. Cell-adhesion-dependent influences on genomic instability and carcinogenesis. Curr Opin Cell Biol. 1998;10(5):647–53. https://doi.org/10.1016/s0955-0674(98)80041-0.
Vaisar T, Kassim SY, Gomez IG, Green PS, Hargarten S, Gough PJ, et al. MMP-9 sheds the beta2 integrin subunit (CD18) from macrophages. Mol Cell Proteomics. 2009;8(5):1044–60. https://doi.org/10.1074/mcp.M800449-MCP200.
Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48(3):222–72. https://doi.org/10.3109/10409238.2013.770819.
Veeraraghavan J, Natarajan M, Herman TS, Aravindan N. Curcumin-altered p53-response genes regulate radiosensitivity in p53-mutant Ewing’s sarcoma cells. Anticancer Res. 2010;30(10):4007–15.
Veeraraghavan J, Natarajan M, Aravindan S, Herman TS, Aravindan N. Radiation-triggered tumor necrosis factor (TNF) alpha-NFkappaB cross-signaling favors survival advantage in human neuroblastoma cells. J Biol Chem. 2011;286(24):21588–600. https://doi.org/10.1074/jbc.M110.193755.
Xu Y, Wang X, Guo B, Wang D, Kalvakolanu DV, Chen X, et al. Nonviral delivery of GRIM-19 gene inhibits tumor growth with reduced local and systemic complications. Hum Gene Ther. 2019;30(11):1419–30. https://doi.org/10.1089/hum.2019.134.
Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology (bethesda). 2013;28(6):391–403. https://doi.org/10.1152/physiol.00029.2013.
Yang JM, Xu Z, Wu H, Zhu H, Wu X, Hait WN. Overexpression of extracellular matrix metalloproteinase inducer in multidrug resistant cancer cells. Mol Cancer Res. 2003;1(6):420–7.
Yang CC, Lin CC, Hsiao LD, Yang CM. Galangin inhibits thrombin-induced MMP-9 expression in SK-N-SH cells via protein kinase-dependent NF-kappaB phosphorylation. International journal of molecular sciences. 2018;19(12). https://doi.org/10.3390/ijms19124084.
Yousefi M, Ghaffari SH, Soltani BM, Nafissi S, Momeny M, Zekri A, et al. Therapeutic efficacy of silibinin on human neuroblastoma cells: Akt and NF-kappaB expressions may play an important role in silibinin-induced response. Neurochem Res. 2012;37(9):2053–63. https://doi.org/10.1007/s11064-012-0827-9.
Zenitani M, Nojiri T, Hosoda H, Kimura T, Uehara S, Miyazato M, et al. Chemotherapy can promote liver metastasis by enhancing metastatic niche formation in mice. J Surg Res. 2018;224:50–7. https://doi.org/10.1016/j.jss.2017.11.050.
Zhang F, Wang Z, Fan Y, Xu Q, Ji W, Tian R, et al. Elevated STAT3 signaling-mediated upregulation of MMP-2/9 confers enhanced invasion ability in multidrug-resistant breast cancer cells. Int J Mol Sci. 2015;16(10):24772–90. https://doi.org/10.3390/ijms161024772.
Funding
This study is funded by the Department of Radiation Oncology at OUHSC, National Institutes of Health, NIH-P20GM103639, and Oklahoma Center for the Advancement of Science and Technology, OCAST-HR19-045.
Author information
Authors and Affiliations
Contributions
NA contributed to the conception and design of the experiments. DS, SA, RM, MN, and NA performed the experiments and contributed to the acquisition of the data. NA, SA, and MN contributed to data analysis and interpretation of the data. NA and DS drafted the manuscript, and MN helped in revising it critically. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Radiation-induced NFκB is required and adequate for the maintenance of MMP9 in NB.
• RT-triggered NFκB-dependent MMP9 actuates a second-signaling feedback to NFκB.
• MMP9-NFκB second signaling feedback is mediated by MMP9-dependent activation of IKKβ and ERK activity.
• NFκB-MMP9-NFκB feedback–dependent acquired maintenance of MMP9 after therapeutic pressure could contribute heavily for tumor evolution.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Somasundaram, D.B., Aravindan, S., Major, R. et al. MMP-9 reinforces radiation-induced delayed invasion and metastasis of neuroblastoma cells through second-signaling positive feedback with NFκB via both ERK and IKK activation. Cell Biol Toxicol 39, 1053–1076 (2023). https://doi.org/10.1007/s10565-021-09663-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-021-09663-4