Abstract
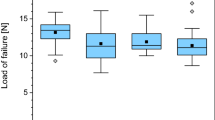
Thermodisinfection of human femoral heads from living donors harvested during hip joint replacement is an established processing procedure. This study was designed to examine the influence of heat sterilization on pull out strength of cancellous bone and storage at different temperatures up to 2 years since we had previously studied the storage of unprocessed cancellous bone. Porcine cancellous bone resembling human bone structure was obtained from 140 proximal humerus of 6–8 months old piglets. Pull out strength of screws after thermodisinfection was compared with unprocessed cancellous bone and tested immediately and after 6, 12 and 24 months of storage at −20 and −80 °C. A three-way ANOVA was performed and significance level was 5 %. The thermodisinfected bone showed a pull out force of 2729 N (1657–3568 N). The reduction of pull out strength compared with unprocessed bone over all periods of storage was 276 N on average with 95 % confidence interval ranging from 166 N to 389 N (p < 0.0001). Different freezing temperatures did not influence this mechanic property within 24 months storage and showed no difference compared with fresh frozen bone. Thermodisinfection of cancellous bone preserves tensile strength necessary for clinical purposes. The storage at −20 °C for at least 2 years did not show relevant decrease of pull out strength compared with −80 °C without difference between thermodisinfected and fresh frozen bone. The increase of the storage temperature to −20 °C for at least 2 years should be considered.





Similar content being viewed by others
References
Bormann N, Pruss A, Schmidmaier G, Wildemann B (2010) In vitro testing of the osteoinductive potential of different bony allograft preparations. Arch Orthop Trauma Surg 130:143–149
Bos GD, Goldberg M, Gordon NH et al (1985) The long-term fate of fresh and frozen orthotopic bone allografts in genetically defined rats. Clin Orthop Relat Res 197:247–254
Burwell RG, Gowland G (1960) Lymph node reactivity to homografts of cancellous bone. Nature 188:159–160
Burwell RG, Gowland G, Dexter F (1963) Studies in the transplantation of Bone VI. Further observations concerning the antigenicity of homologous cortical and cancellous bone. J Bone Joint Surg [Br] 45-B:597–608
Buttaro MA, Costantini J, Comba F, Piccaluga F (2012) The use of femoral struts and impacted cancellous bone allograft in patients with severe femoral bone loss who undergo revision total hip replacement. J Bone Joint Surg [Br] 94-B:167–172
Center of Disease Control and Prevention (1988) Transmission of HIV through bone transplantation: case report and public health recommendations. MMWR 37:597–599
Center of Disease Control and Prevention (2002) Allograft associated bacterial infections. MMWR 51:207–210
Cornwell KG, Lei P, Andreadis ST, Pins GD (2007) Crosslinking of discrete self-assembled collagen threads: effects on mechanical strength and cell-matrix interactions. J Biomed Mater Res A 80:362–371
Delloye C, Cornu O, Druez V, Barbier O (2007) Bone allografts. J Bone Joint Surg 89-B:574–579
Donk S, Weernink T, Buma P, Aspenberg P, Sloof T, Schreurs BW (2003) Rinsing morselized allografts improves bone and tissue ingrowth. Clin Orthop Relat Res 408:302–310
Donnelly E (2011) Methods for assessing bone quality: a review. Clin Orthop Relat Res 469:2128–2138
Fantner GE, Birkedal H, Kindt JH et al (2004) Influence of the degradation of the organic matrix on the microscopic fracture behavior of trabecular bone. Bone 35:1013–1022
Fölsch C, Mittelmeier W, Bilderbeek U, Timmesfeld N, von Garrel T, Peter Matter H (2012) Effect of storage temperature on allograft bone. Transfus Med Hemother 39:36–40
Fosse L, Ronningen H, Benum P, Lydersen S, Sandven RB (2006) Factors affecting stiffness properties in impacted morsellized bone used in revision hip surgery: an experimental in vitro study. J Biomed Mater Res A 78:423–431
Fröhlich M, Grayson WL, Wan LQ, Marolt D, Drobnic M, Vunjak-Novakovic G (2008) Tissue engineered bone grafts: biological requirements, tissue culture and clinical relevance. Curr Stem Cell Res Ther 3:254–264
Gallie WE (1918) The use of boiled bone in operative surgery. Am Orthop Surg 16:373
Hamer AJ, Strachan JR, Black MM, Ibbotson CJ, Stockley I, Elson RA (1996) Biomechanical properties of cortical allograft bone using a new method of bone strength measurement. J Bone Joint Surg [Br] 78-B:363–368
Hart MM, Campbell ED, Kartur MG (1986) Bone banking. Clin Orthop Relat Res 206:295–300
Heyligers IC, Klein-Nulend J (2005) Detection of living cells in non-processed but deep frozen bone allografts. Cell Tissue Bank 6:25–31
Holzmann P, Niculescu-Morzsa E, Zwickl H et al (2010) Investigation of bone allografts representing different steps of the bone bank procedure using the CAM-model. ALTEX 27:97–103
Kaplan SJ, Hayes WC, Stone JL, Beaupré GS (1985) Tensile strength of bovine trabecular bone. J Biomech 18:723–727
Keaveny TM, Wachtel EF, Ford CM (1994) Hayes WC (1994) Differences between tensile and compressive strengths of bovine tibial trabecular bone dependent on modulus. J Biomech 27:1137–1146
Kopperdahl DL, Keaveny TM (1998) Yield strain behavior of trabecular bone. J Biomech 31:601–608
Langer F, Czitrom A, Pritzker KP, Gross AE (1975) The immunogenicity of fresh frozen allogeneic bone. J Bone Joint Surg [Am] 57-A:216–220
Linde F, Sörensen HCF (1993) The Effect of different storage methods on the mechanical properties of trabecular bone. J Biomechanics 26:1249–1252
Matter HP, Garrel T, Bilderbeek U, Mittelmeier W (2001) Biomechanical examinations of cancellous bone concerning the influence of duration and temperature cryopreservation. J Biomed Mater Res 55:40–44
McNamara I, Deshpande S, Porteous M (2010) Impaction grafting of the acetabulum with a mixture of frozen, ground irradiated bone graft and porous synthetic bone substitute (Apapore 60). J Bone Joint Surg [Br] 92-B:617–623
Mejdahl S, Hansen CA, Skjodt H, Reimann I (1998) Human bone bank allografts stimulate bone resorption and inhibit proliferation in cultures of human osteoblast-like cells. Acta Orthop Scand 69:63–68
Ochs BG, Schmid U, Rieth J, Ateschrang A, Weise K, Ochs U (2008) Acetabular bone reconstruction in revision arthroplasty. J Bone Joint Surg [Br] 90-B:1164–1171
Palmer SH, Gibbons CLM, Athanasou NA (1999) The pathology of bone allograft. J Bone Joint Surg [Br] 81-B(2):333–335
Pelker RR, Friedlaender GE, Markham TC, Panjabi MM, Moen CJ (1984) Effects of freeze-drying on the biomechanical properties of rat bone. J Orthop Res 1:405–411
Piedra C, Vicario C, de Acuna LR, Garcia-Moreno C, Traba ML, Arlandis S, Marco F, Lopez-Duran L (2008) Osteoinductive effect of bone bank allografts on human osteoblasts in culture. J Orthop Res 26:200–207
Pruss A, Kao M, von Garrel T et al (2003a) Virus inactivation in bone tissue transplants (femoral heads) by moist heat with the Marburg bone bank system. Biologicals 31:75–82
Pruss A, Seibold M, Benedix F et al (2003b) Validation of the Marburg bone bank system for thermodisinfection of allogeneic femoral head transplants using selected bacteria, fungi and spores. Biologicals 31:287–294
Ranki A, Valle SL, Krohn M et al (1987) Long latency precedes overt seroconversion in sexually transmitted human-immunodeficiency-virus infection. Lancet 330(8559):589–593
Reikeras O, Reinholt FP, Zinöcker S, Shegarfi H, Rolstad B (2011) Healing of long-term frozen orthotopic bone allografts is not affected by MHC differences between donor and recipient. Clin Orthop Relat Res 469:1479–1486
Rogers KD, Daniels P (2002) An X-ray diffraction study of the effects of heat treatment on bone mineral microstructure. Biomaterials 23:2577–2585
Sakai T, Ohzono K, Nishii T, Miki H, Takao M, Sugano N (2010) Grafting with hydroxyapatite granules for defects of acetabular bone at Revision total hip replacement. J Bone Joint Surg [Br] 92-B:1215–1221
Salai M, Brosh T, Keller N, Perelman M, Dudkiewicz I (2000) The effects of prolonged cryopreservation on the biomechanical properties of bone allografts: a microbiological, histological and mechanical study. Cell Tissue Bank 1:69–73
Shimuzu K, Masumi S, Yano H, Fukunaga T (1999) Revascularization and new bone formation in heat-treated bone grafts. Arch Orthop Trauma Surg 119:57–61
Shin S, Yano H, Fukunaga T et al (2005) Biomechanical properties of heat treated bone grafts. Arch Orthop Trauma Surg 125:1–5
Shirakawa H, Furusawa K, Fukui A, Tadano S, Sasaki N (2013) Changes in the viscoelastic properties of cortical bone by selective degradation of matrix protein. J Biomech 46:696–701
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Thoren K, Aspenberg P, Thorngren KG (1995) Lipid extracted bank bone. Clin Orthop Relat Res 311:232–246
Tomford WW (1995) Transmission of disease through transplantation of musculoskeletal allografts. J Bone Joint Surg [Am] 77-A:1742–1754
Tomford WW, Doppelt SH, Mankin HJ, Friedlaender GE (1983) 1983 Bone Bank Procedures. Clin Orthop Relat Res 174:15–21
Tomford WW, Ploetz JE, Mankin HJ (1986) Bone allografts of femoral heads: procurement and storage. J Bone Joint Surg [Am] 68-A:534–537
Urist MR, Silverman BF, Büring K, Dubuc FL (1967) The bone induction principle. Clin Orthop 53:243–283
Vastel L, Meunier A, Siney H, Sedel L, Courpied JP (2004) Effect of different sterilization processing methods on the mechanical properties of human cancellous bone allografts. Biomaterials 25:2105–2110
Viquet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporosis Int 17:319–336
Wang X, Bank R, TeKoppele J, Hubbard G, Athanasiou K, Agrawal C (2000) Effect of collagen denaturation on the toughness of bone. Clin Orthop Relat Res 371:228–239
Yamashita J, Furman BR, Rawis HR, Wang X, Agrawal M (2001) The use of dynamic mechanical analysis to assess the viscoelastic properties of human cortical bone. J Biomed Mater Res 58:47–53
Yamashita J, Li X, Furman BR, Rawls HR, Wang X, Agrawal CM (2002) Collagen and bone viscoelasticity: a dynamic mechanical analysis. J Biomed Mater Res 63:31–36
Zioupos P, Currey J, Hamer A (1999) The role of collagen in the declining mechanical properties of aging human cortical bone. J Biomed Mater Res 45:108–116
Acknowledgments
We are particularly grateful for the support offered during the biomechanical examination to Prof. Dr. Ing. M. Niebert, Fachhochschule Gießen/Friedberg. Department Biomedizin und Technologie, Gießen and to Prof. Dr. H. Stürz, Director of the Orthopaedic University Clinic, Gießen.
Conflict of interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fölsch, C., Mittelmeier, W., von Garrel, T. et al. Influence of thermodisinfection and duration of cryopreservation at different temperatures on pull out strength of cancellous bone. Cell Tissue Bank 16, 73–81 (2015). https://doi.org/10.1007/s10561-014-9442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-014-9442-0