Abstract
Purpose
Attenuated vasodilatation of small arteries is a hallmark feature of hypertension. Salusin-β, which is a TOR2A gene product and an important vasoactive peptide, has a close relationship with cardiovascular disease. This study aimed to determinate the roles of salusin-β in vasodilatation, and its signal pathways in Wistar–Kyoto rats (WKY) and spontaneously hypertensive rats (SHR).
Methods
Isometric tension experiments were performed. Vasodilatation was induced by acetylcholine (ACh) or sodium nitroprusside (SNP).
Results
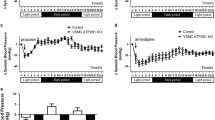
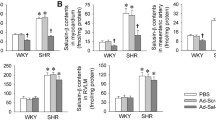
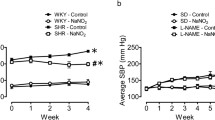
Plasma salusin-β levels and their protein expressions in coronary artery (CA), mesenteric artery (MA), and pulmonary artery (PA) of SHR were much higher than that of WKY. Intravenous injection of salusin-β increased arterial blood pressure in SHR, while anti-salusin-β IgG decreased it. Salusin-β further deteriorated, while anti-salusin-β IgG improved, the attenuated ACh-induced relaxation, the decreased nitric oxide (NO) level, and endothelial nitric oxide synthase (eNOS) activity in arteries of SHR, and salusin-β had no significant effect on SNP-induced relaxation. The NAD(P)H oxidase activity and reactive oxygen species (ROS) level in arteries of SHR were much higher than that of WKY, which was further increased by salusin-β but reduced by anti-salusin-β IgG. ROS scavenger NAC or antioxidant apocynin significantly inhibited, while SOD inhibitor DETC aggravated, the effects of salusin-β, and the eNOS inhibitor L-NAME inhibited the effects of anti-salusin-β IgG.
Conclusions
These results indicated that enhanced salusin-β activity is involved in attenuated endothelium-dependent vasodilatation pathogenesis in SHR by activating NAD(P)H oxidase derived ROS generation and inhibiting eNOS activation and NO release.








Similar content being viewed by others
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6:41–50.
Gargiulo R, Suhail F, Lerma EV. Hypertension and chronic kidney disease. Dis Mon. 2015;61:387–95.
Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–52.
Virdis A, Bacca A, Colucci R, Duranti E, Fornai M, Materazzi G, et al. Endothelial dysfunction in small arteries of essential hypertensive patients: role of cyclooxygenase-2 in oxidative stress generation. Hypertension. 2013;62:337–44.
Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–62.
Wanstall JC, Homer KL, Doggrell SA. Evidence for, and importance of, cGMP-independent mechanisms with NO and NO donors on blood vessels and platelets. Curr Vasc Pharmacol. 2005;3:41–53.
Yuan XJ, Tod ML, Rubin LJ, Blaustein MP. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc Natl Acad Sci U S A. 1996;93:10489–94.
Raffetto JD, Calanni F, Mattana P, Khalil RA. Sulodexide Promotes Arterial Relaxation via Endothelium-Dependent Nitric Oxide-Mediated Pathway. Biochem Pharmacol. 2019;166:347–356.
Han Y, Cho YE, Ayon R, Guo R, Youssef KD, Pan M, et al. SGLT inhibitors attenuate NO-dependent vascular relaxation in the pulmonary artery but not in the coronary artery. Am J Phys Lung Cell Mol Phys. 2015;309:L1027–36.
Teixeira-da-Silva JJ, Nunes-Moreira HS, Silva CO, Lahlou S, Naro F, Xavier FE, et al. Chronic administration of sildenafil improves endothelial function in spontaneously hypertensive rats by decreasing COX-2 expression and oxidative stress. Life Sci. 2019;225:29–38.
Zhang F, Tang H, Sun S, Luo Y, Ren X, Chen A, et al. Angiotensin-(1-7) induced vascular relaxation in spontaneously hypertensive rats. Nitric Oxide. 2019;88:1–9.
Zhang F, Xu Y, Pan Y, Sun S, Chen A, Li P, et al. Effects of angiotensin-(1-7) and angiotensin II on acetylcholine-induced vascular relaxation in spontaneously hypertensive rats. Oxidative Med Cell Longev. 2019;2019:6512485.
Shichiri M, Ishimaru S, Ota T, Nishikawa T, Isogai T, Hirata Y. Salusins: newly identified bioactive peptides with hemodynamic and mitogenic activities. Nat Med. 2003;9:1166–72.
Kolakowska U, Kuroczycka-Saniutycz E, Wasilewska A, Olanski W. Is the serum level of salusin-beta associated with hypertension and atherosclerosis in the pediatric population? Pediatr Nephrol. 2015;30:523–31.
Sato K, Watanabe R, Itoh F, Shichiri M, Watanabe T. Salusins: potential use as a biomarker for atherosclerotic cardiovascular diseases. Int J Hypertens. 2013;2013:965140.
Suzuki N, Shichiri M, Tateno T, Sato K, Hirata Y. Distinct systemic distribution of salusin-alpha and salusin-beta in the rat. Peptides. 2011;32:805–10.
Sato K, Sato T, Susumu T, Koyama T, Shichiri M. Presence of immunoreactive salusin-beta in human plasma and urine. Regul Pept. 2009;158:63–7.
Watanabe T, Nishio K, Kanome T, Matsuyama TA, Koba S, Sakai T, et al. Impact of salusin-alpha and -beta on human macrophage foam cell formation and coronary atherosclerosis. Circulation. 2008;117:638–48.
Shichiri M, Nonaka D, Lee LJ, Tanaka K. Identification of the salusin-beta receptor using proteoliposomes embedded with endogenous membrane proteins. Sci Rep. 2018;8:17865.
Koya T, Miyazaki T, Watanabe T, Shichiri M, Atsumi T, Kim-Kaneyama JR, et al. Salusin-beta accelerates inflammatory responses in vascular endothelial cells via NF-kappaB signaling in LDL receptor-deficient mice in vivo and HUVECs in vitro. Am J Physiol Heart Circ Physiol. 2012;303:H96–105.
Sato K, Fujimoto K, Koyama T, Shichiri M. Release of salusin-beta from human monocytes/macrophages. Regul Pept. 2010;162:68–72.
Zhang LL, Ding L, Zhang F, Gao R, Chen Q, Li YH, et al. Salusin-beta in rostral ventrolateral medulla increases sympathetic outflow and blood pressure via superoxide anions in hypertensive rats. J Hypertens. 2014;32:1059–67 discussion 67.
Chen WW, Sun HJ, Zhang F, Zhou YB, Xiong XQ, Wang JJ, et al. Salusin-beta in paraventricular nucleus increases blood pressure and sympathetic outflow via vasopressin in hypertensive rats. Cardiovasc Res. 2013;98:344–51.
Sun HJ, Zhang LL, Fan ZD, Chen D, Zhang L, Gao XY, et al. Superoxide anions involved in sympathoexcitation and pressor effects of salusin-beta in paraventricular nucleus in hypertensive rats. Acta Physiol (Oxford). 2014;210:534–45.
Xie FJ, Chai C, Zhu P, Li B, Cai HY, Lu Y, et al. The cardiovascular functions of salusin-beta mediated by muscarinic receptors, glutamate receptors or L-type calcium channels within the rostral ventrolateral medulla of rats. Microsc Res Tech. 2017;80:812–9.
Li HB, Qin DN, Suo YP, Guo J, Su Q, Miao YW, et al. Blockade of Salusin-beta in hypothalamic paraventricular nucleus attenuates hypertension and cardiac hypertrophy in salt-induced hypertensive rats. J Cardiovasc Pharmacol. 2015;66:323–31.
Sun HJ, Chen D, Wang PY, Wan MY, Zhang CX, Zhang ZX, et al. Salusin-beta is involved in diabetes mellitus-induced endothelial dysfunction via degradation of peroxisome proliferator-activated receptor gamma. Oxidative Med Cell Longev. 2017;2017:6905217.
Zhao MX, Zhou B, Ling L, Xiong XQ, Zhang F, Chen Q, et al. Salusin-beta contributes to oxidative stress and inflammation in diabetic cardiomyopathy. Cell Death Dis. 2017;8:e2690.
Sun HJ, Liu TY, Zhang F, Xiong XQ, Wang JJ, Chen Q, et al. Salusin-beta contributes to vascular remodeling associated with hypertension via promoting vascular smooth muscle cell proliferation and vascular fibrosis. Biochim Biophys Acta. 1852;2015:1709–18.
Li HB, Qin DN, Cheng K, Su Q, Miao YW, Guo J, et al. Central blockade of salusin beta attenuates hypertension and hypothalamic inflammation in spontaneously hypertensive rats. Sci Rep. 2015;5:11162.
Li HB, Yu XJ, Bai J, Su Q, Wang ML, Huo CJ, et al. Silencing salusin beta ameliorates heart failure in aged spontaneously hypertensive rats by ROS-relative MAPK/NF-kappaB pathways in the paraventricular nucleus. Int J Cardiol. 2019;280:142–51.
Ren X, Zhang F, Zhao M, Zhao Z, Sun S, Fraidenburg DR, et al. Angiotensin-(1-7) in paraventricular nucleus contributes to the enhanced cardiac sympathetic afferent reflex and sympathetic activity in chronic heart failure rats. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 2017;42:2523-39.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40.
Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014;64:924–8.
Watanabe T, Sato K, Itoh F, Iso Y, Nagashima M, Hirano T, et al. The roles of salusins in atherosclerosis and related cardiovascular diseases. J Am Soc Hypertens. 2011;5:359–65.
Zhou CH, Pan J, Huang H, Zhu Y, Zhang M, Liu L, et al. Salusin-beta, but not salusin-alpha, promotes human umbilical vein endothelial cell inflammation via the p38 MAPK/JNK-NF-kappaB pathway. PLoS One. 2014;9:e107555.
Ren XS, Ling L, Zhou B, Han Y, Zhou YB, Chen Q, et al. Silencing salusin-beta attenuates cardiovascular remodeling and hypertension in spontaneously hypertensive rats. Sci Rep. 2017;7:43259.
Matsumura K. Salusin and central regulation of blood pressure in hypertension. J Hypertens. 2014;32:981–2.
Xu T, Zhang Z, Liu T, Zhang W, Liu J, Wang W, et al. Salusin-beta contributes to vascular inflammation associated with pulmonary arterial hypertension in rats. J Thorac Cardiovasc Surg. 2016;152:1177–87.
Sun HJ, Zhao MX, Ren XS, Liu TY, Chen Q, Li YH, et al. Salusin-beta promotes vascular smooth muscle cell migration and intimal hyperplasia after vascular injury via ROS/NFkappaB/MMP-9 pathway. Antioxid Redox Signal. 2016;24:1045–57.
Sun HJ, Zhao MX, Liu TY, Ren XS, Chen Q, Li YH, et al. Salusin-beta induces foam cell formation and monocyte adhesion in human vascular smooth muscle cells via miR155/NOX2/NFkappaB pathway. Sci Rep. 2016;6:23596.
Zhu X, Zhou Y, Cai W, Sun H, Qiu L. Salusin-beta mediates high glucose-induced endothelial injury via disruption of AMPK signaling pathway. Biochem Biophys Res Commun. 2017;491:515–21.
Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98.
Wang Z, Takahashi T, Saito Y, Nagasaki H, Ly NK, Nothacker HP, et al. Salusin beta is a surrogate ligand of the mas-like G protein-coupled receptor MrgA1. Eur J Pharmacol. 2006;539:145–50.
Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–57.
Esfahani M, Saidijam M, Najafi R, Goodarzi MT, Movahedian A. The effect of salusin-beta on expression of pro- and anti-inflammatory cytokines in human umbilical vein endothelial cells (HUVECs). ARYA Atheroscler. 2018;14:1–10.
Panieri E, Santoro MM. ROS signaling and redox biology in endothelial cells. Cell Mol Life Sci. 2015;72:3281–303.
Sag CM, Schnelle M, Zhang J, Murdoch CE, Kossmann S, Protti A, et al. Distinct regulatory effects of myeloid cell and endothelial cell NAPDH oxidase 2 on blood pressure. Circulation. 2017;135:2163–77.
Yin Y, Zhou Z, Liu W, Chang Q, Sun G, Dai Y. Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int J Biochem Cell Biol. 2017;84:22–34.
Nagashima M, Watanabe T, Shiraishi Y, Morita R, Terasaki M, Arita S, et al. Chronic infusion of salusin-alpha and -beta exerts opposite effects on atherosclerotic lesion development in apolipoprotein E-deficient mice. Atherosclerosis. 2010;212:70–7.
Funding
This work was sponsored by the National Natural Science Foundation of China [31571168, 81470538, 81770059, and 81770050], Qing Lan Project of Jiangsu Province of China, and the Open Project of the State Key Laboratory of Respiratory Disease (SKLRD-OP-201911).
Author information
Authors and Affiliations
Contributions
All authors contributed to the work in this paper. Y.H. and H.T. conceived and designed the experiments. S.S., F.Z., Y.X., and Y.P. performed the experiments. S.S. and A.D.C analyzed the data. Y.H. and H.T. wrote the manuscript. J.W. and H.T. provided intellectual suggestions and critically revised the article.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All animal procedures were reviewed and approved by Nanjing Medical University Experimental Animal Care and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication, 8th edition, 2011).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, S., Zhang, F., Pan, Y. et al. A TOR2A Gene Product: Salusin-β Contributes to Attenuated Vasodilatation of Spontaneously Hypertensive Rats. Cardiovasc Drugs Ther 35, 125–139 (2021). https://doi.org/10.1007/s10557-020-06983-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-06983-1