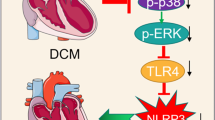
Abstract
Purpose
Ticagrelor, a P2Y12 receptor antagonist, and dapagliflozin, a sodium–glucose-cotransporter-2 inhibitor, suppress the activation of the NLRP3 inflammasome. The anti-inflammatory effects of dapagliflozin depend on AMPK activation. Also, ticagrelor can activate AMPK. We assessed whether dapagliflozin and ticagrelor have additive effects in attenuating the progression of diabetic cardiomyopathy in T2DM mice.
Methods
Eight-week-old BTBR and wild-type mice received no drug, dapagliflozin (1.5 mg/kg/day), ticagrelor (100 mg/kg/day), or their combination for 12 weeks. Heart function was evaluated by echocardiography and heart tissue samples were assessed for fibrosis, apoptosis, qRT-PCR, and immunoblotting.
Results
Both drugs attenuated the progression of diabetic cardiomyopathy as evident by improvements in left ventricular end-systolic and end-diastolic volumes and left ventricular ejection fraction, which were further improved by the combination. Both drugs attenuated the activation of the NOD-like receptor 3 (NLRP3) inflammasome and fibrosis. The effect of the combination was significantly greater than each drug alone on myocardial tissue necrotic factorα (TNFα) and interleukin-6 (IL-6) levels, suggesting additive effects. The combination had also a greater effect on ASC, collagen-1, and collagen-3 mRNA levels than each drug alone. While both drugs activated adenosine mono-phosphate kinase (AMPK), only dapagliflozin activated mTOR and increased RICTOR levels. Moreover, only dapagliflozin decreased myocardial BNP and Caspase-1 mRNA levels, and the effects of dapagliflozin on NLRP3 and collagen-3 mRNA levels were significantly greater than those of ticagrelor.
Conclusions
Both dapagliflozin and ticagrelor attenuated the progression of diabetic cardiomyopathy, the activation of the NLRP3 inflammasome, and fibrosis in BTBR mice with additive effects of the combination. While both dapagliflozin and ticagrelor activated AMPK, only dapagliflozin activated mTOR complex 2 (mTORC2) in hearts of BTBR mice.











Similar content being viewed by others
References
Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323.
Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309–20.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019.
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–57.
Birnbaum Y, Birnbaum GD, Birnbaum I, Nylander S, Ye Y. Ticagrelor and rosuvastatin have additive cardioprotective effects via adenosine. Cardiovasc Drugs Ther. 2016;30(6):539–50.
Birnbaum Y, Tran D, Chen H, Nylander S, Sampaio L, Ye Y. Ticagrelor improves remodeling, reduces apoptosis, inflammation and fibrosis and increases the number of progenitor stem cells after myocardial infarction in a rat model of ischemia reperfusion. Cell Physiol Biochem. 2019;53(6):961–81.
Nanhwan MK, Ling S, Kodakandla M, Nylander S, Ye Y, Birnbaum Y. Chronic treatment with ticagrelor limits myocardial infarct size: an adenosine and cyclooxygenase-2-dependent effect. Arterioscler Thromb Vasc Biol. 2014;34(9):2078–85.
Ye Y, Birnbaum GD, Perez-Polo JR, Nanhwan MK, Nylander S, Birnbaum Y. Ticagrelor protects the heart against reperfusion injury and improves remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2015;35(8):1805–14.
Ye Y, Nylander S, Birnbaum Y. Unraveling the interaction of aspirin, ticagrelor, and rosuvastatin on the progression of atherosclerosis and inflammation in diabetic mice. Cardiovasc Drugs Ther. 2017;31(5–6):489–500.
Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–53.
Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61(1):21–8.
Athithan L, Gulsin GS, McCann GP, Levelt E. Diabetic cardiomyopathy: pathophysiology, theories and evidence to date. World J Diabetes. 2019;10(10):490–510.
Ye Y, Bajaj M, Yang HC, Perez-Polo JR, Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31(2):119–32.
Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13(1):e006277.
Ye Y, Jia X, Bajaj M, Birnbaum Y. Dapagliflozin attenuates Na(+)/H(+) exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther. 2018;32(6):553–8.
Moustafa Ahmed Y, Shehata Messiha BA, El-Sayed El-Daly M, Abo-Saif AA. Effects of ticagrelor, empagliflozin and tamoxifen against experimentally-induced vascular reactivity defects in rats in vivo and in vitro. Pharmacol Rep. 2019;71(6):1034–43.
Vilahur G, Gutierrez M, Casani L, Lambert C, Mendieta G, Ben-Aicha S, et al. P2Y12 antagonists and cardiac repair post-myocardial infarction: global and regional heart function analysis and molecular assessments in pigs. Cardiovasc Res. 2018;114(14):1860–70.
Sun W, Zeng C, Liu S, Fu J, Hu L, Shi Z, et al. Ageratina adenophora induces mice hepatotoxicity via ROS-NLRP3-mediated pyroptosis. Sci Rep. 2018;8(1):16032.
Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315(6):H1553–H68.
Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation. 2019;139(22):2516–27.
Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, et al. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019;394(10204):1169–80.
Aymerich I, Foufelle F, Ferre P, Casado FJ, Pastor-Anglada M. Extracellular adenosine activates AMP-dependent protein kinase (AMPK). J Cell Sci. 2006;119(Pt 8):1612–21.
Peng Z, Luo R, Xie T, Zhang W, Liu H, Wang W, et al. Erythrocyte adenosine A2B receptor-mediated AMPK activation: a missing component counteracting CKD by promoting oxygen delivery. J Am Soc Nephrol. 2019;30(8):1413–24.
Ruan CC, Kong LR, Chen XH, Ma Y, Pan XX, Zhang ZB, et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab. 2018;28(3):476–89 e5.
Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, et al. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol. 2009;53(5):373–8.
Li T, Jiang S, Yang Z, Ma Z, Yi W, Wang D, et al. Targeting the energy guardian AMPK: another avenue for treating cardiomyopathy? Cell Mol Life Sci. 2017;74(8):1413–29.
Yan Y, Zhou XE, Xu HE, Melcher K. Structure and physiological regulation of AMPK. Int J Mol Sci. 2018;19(11).
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. beta-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016.
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204.
Fassett JT, Hu X, Xu X, Lu Z, Zhang P, Chen Y, et al. AMPK attenuates microtubule proliferation in cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2013;304(5):H749–58.
Javadov S, Rajapurohitam V, Kilic A, Zeidan A, Choi A, Karmazyn M. Anti-hypertrophic effect of NHE-1 inhibition involves GSK-3beta-dependent attenuation of mitochondrial dysfunction. J Mol Cell Cardiol. 2009;46(6):998–1007.
Barreto-Torres G, Hernandez JS, Jang S, Rodriguez-Munoz AR, Torres-Ramos CA, Basnakian AG, et al. The beneficial effects of AMP kinase activation against oxidative stress are associated with prevention of PPARalpha-cyclophilin D interaction in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2015;308(7):H749–58.
Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ Res. 2014;114(3):549–64.
Suhara T, Baba Y, Shimada BK, Higa JK, Matsui T. The mTOR signaling pathway in myocardial dysfunction in type 2 diabetes mellitus. Curr Diab Rep. 2017;17(6):38.
Zhang D, Contu R, Latronico MV, Zhang J, Rizzi R, Catalucci D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120(8):2805–16.
Song X, Kusakari Y, Xiao CY, Kinsella SD, Rosenberg MA, Scherrer-Crosbie M, et al. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299(6):C1256–66.
Xu X, Kobayashi S, Timm D, Huang Y, Zhao F, Shou W, et al. Enhanced mTOR complex 1 signaling attenuates diabetic cardiac injury in OVE26 mice. FASEB J. 2019;33(11):12800–11.
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450(7170):736–40.
Zhu Y, Soto J, Anderson B, Riehle C, Zhang YC, Wende AR, et al. Regulation of fatty acid metabolism by mTOR in adult murine hearts occurs independently of changes in PGC-1alpha. Am J Physiol Heart Circ Physiol. 2013;305(1):H41–51.
Sciarretta S, Forte M, Frati G, Sadoshima J. New insights into the role of mTOR signaling in the cardiovascular system. Circ Res. 2018;122(3):489–505.
Tang H, Wu K, Wang J, Vinjamuri S, Gu Y, Song S, et al. Pathogenic role of mTORC1 and mTORC2 in pulmonary hypertension. JACC Basic Transl Sci. 2018;3(6):744–62.
Hu Z, Wang F, Wu Z, Gu H, Dong N, Jiang X, et al. FOXO3a-dependent up-regulation of Mxi1-0 promotes hypoxia-induced apoptosis in endothelial cells. Cell Signal. 2018;51:233–42.
Liu MH, Li GH, Peng LJ, Qu SL, Zhang Y, Peng J, et al. PI3K/Akt/FoxO3a signaling mediates cardioprotection of FGF-2 against hydrogen peroxide-induced apoptosis in H9c2 cells. Mol Cell Biochem. 2016;414(1–2):57–66.
Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, et al. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy. 2015;11(7):1146–60.
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335(6076):1638–43.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30(2):214–26.
Birnbaum Y, Nanhwan MK, Ling S, Perez-Polo JR, Ye Y, Bajaj M. PTEN upregulation may explain the development of insulin resistance and type 2 diabetes with high dose statins. Cardiovasc Drugs Ther. 2014;28(5):447–57.
Funding
The study was funded by an investigator-initiated grant from AstraZeneca and the John S. Dunn Chair in Cardiology Research and Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Huan Chen declares no conflict of interest. Da Tran declares no conflict of interest. Hsiu-Chiung Yang is an employee of AstraZeneca. Sven Nylander is an employee of AstraZeneca. Yochai Birnbaum received a research grant from AstraZeneca. Yumei Ye received a research grant from AstraZeneca.
Ethical Statement
The experimental designs and animal care were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH publication no. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Tran, D., Yang, HC. et al. Dapagliflozin and Ticagrelor Have Additive Effects on the Attenuation of the Activation of the NLRP3 Inflammasome and the Progression of Diabetic Cardiomyopathy: an AMPK–mTOR Interplay. Cardiovasc Drugs Ther 34, 443–461 (2020). https://doi.org/10.1007/s10557-020-06978-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-020-06978-y