Abstract
Background
The role of the metabolic syndrome in the etiology of esophageal and gastric cancer is unclear.
Methods
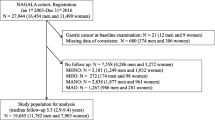
This was a large nationwide cohort study based on data from 11 prospective population-based cohorts in Norway with long-term follow-up, the Cohort of Norway (CONOR) and the third Nord-Trøndelag Health Study (HUNT3). The metabolic syndrome was assessed by objective anthropometric and metabolic biochemical measures and was defined by the presence of at least three of the following five factors: increased waist circumference, elevated triglycerides, low high-density lipoprotein cholesterol, hypertension and high glucose. Newly diagnosed cases of esophageal adenocarcinoma, esophageal squamous-cell carcinoma and gastric adenocarcinoma were identified from the Norwegian Cancer Registry. Hazard ratios (HRs) and 95 % confidence intervals (CIs) were estimated using Cox proportional hazard models with adjustment for potential confounders.
Result
Among 192,903 participants followed up for an average of 10.6 years, 62 developed esophageal adenocarcinoma, 64 had esophageal squamous-cell carcinoma and 373 had gastric adenocarcinoma. The metabolic syndrome was significantly associated with an increased risk of gastric adenocarcinoma (HR 1.44, 95 % CI 1.14–1.82), but not associated with esophageal adenocarcinoma (HR 1.32, 95 % CI 0.77–2.26) or esophageal squamous-cell carcinoma (HR 1.08, 95 % CI 0.64–1.83). Increased waist circumference was associated with an increased HR of esophageal adenocarcinoma (HR 2.48, 95 % CI 1.27–4.85). No significant association was found between any single component of the metabolic syndrome and risk of esophageal squamous-cell carcinoma. High waist circumference (HR 1.71, 95 % CI 1.05–2.80), hypertension (HR 2.41, 95 % CI 1.44–4.03) and non-fasting glucose (HR 1.74, 95 % CI 1.18–2.56) were also related to an increased risk of gastric adenocarcinoma in women, but not in men.
Conclusion
Metabolic syndrome was associated with an increased risk of gastric adenocarcinoma in women. Of the individual components of the metabolic syndrome, high waist circumference was positively associated with risk of esophageal adenocarcinoma. Positive associations were also observed for women between high waist circumference, hypertension, high non-fasting glucose and risk of gastric adenocarcinoma. However, further evidence is warranted due to the limited number of cases and the inability to effectively identify gastric cardia adenocarcinoma.
Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Alberti KG, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
Ford ES, Li CY, Zhao GX (2010) Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2:180–193
Zanchetti A, Hennig M, Baurecht H et al (2007) Prevalence and incidence of the metabolic syndrome in the European Lacidipine Study on Atherosclerosis (ELSA) and its relation with carotid intima-media thickness. J Hypertens 25:2463–2470
Escobedo J, Schargrodsky H, Champagne B et al (2009) Prevalence of the metabolic syndrome in Latin America and its association with sub-clinical carotid atherosclerosis: the CARMELA cross sectional study. Cardiovasc Diabetol 8:52
Beebe-Dimmer JL, Nock NL, Neslund-Dudas C et al (2009) Racial differences in risk of prostate cancer associated with metabolic syndrome. Urology 74:185–190
Hsing AW, Sakoda LC, Chua S Jr (2007) Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr 86:s843–s857
Turati F, Talamini R, Pelucchi C et al (2013) Metabolic syndrome and hepatocellular carcinoma risk. Br J Cancer 108:222–228
Haggstrom C, Stocks T, Rapp K et al (2011) Metabolic syndrome and risk of bladder cancer: prospective cohort study in the metabolic syndrome and cancer project (Me-Can). Int J Cancer 128:1890–1898
Rosato V, Zucchetto A, Bosetti C et al (2011) Metabolic syndrome and endometrial cancer risk. Ann Oncol 22:884–889
Giovannucci E (2007) Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 86:s836–s842
Lindkvist B, Johansen D, Stocks T et al (2014) Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 14:103
Lindkvist B, Almquist M, Bjorge T et al (2013) Prospective cohort study of metabolic risk factors and gastric adenocarcinoma risk in the Metabolic Syndrome and Cancer Project (Me-Can). Cancer Causes Control 24:107–116
Corley DA, Kubo A, Zhao W (2008) Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev 17:352–358
Beddy P, Howard J, McMahon C et al (2010) Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br J Surg 97:1028–1034
Naess O, Sogaard AJ, Arnesen E et al (2008) Cohort profile: cohort of Norway (CONOR). Int J Epidemiol 37:481–485
Krokstad S, Langhammer A, Hveem K et al (2013) Cohort profile: the HUNT study, Norway. Int J Epidemiol 42:968–977
Larsen IK, Smastuen M, Johannesen TB et al (2009) Data quality at the Cancer Registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer 45:1218–1231
Fritz APC, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S (eds) (2000) International classification of diseases for oncology, 3rd edn. World Health Organization, Geneva
Cox DR, Oakes D (1984) Analysis of survival data. Chapman and Hall, London
Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley, New York
MacInnis RJ, English DR, Hopper JL, Giles GG (2006) Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer 118:2628–2631
Steffen A, Schulze MB, Pischon T et al (2009) Anthropometry and esophageal cancer risk in the European Prospective Investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 18:2079–2089
Wu J, Mui WL, Chan Y, Sung J (2007) Obesity is associated with increased transient lower esophageal sphincter relaxation. Gastroenterology 132:A121
Fass R (2008) The pathophysiological mechanisms of GERD in the obese patient. Dig Dis Sci 53:2300–2306
Fornari F, Madalosso CAS, Farre R, Gurski RR, Thiesen V, Callegari-Jacques SM (2010) The role of gastro-oesophageal pressure gradient and sliding hiatal hernia on pathological gastro-oesophageal reflux in severely obese patients. Eur J Gastroenterol Hepatol 22:404–411
Dieudonne MN, Bussiere M, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R (2006) Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem Biophys Res Commun 345:271–279
Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M (2004) Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 363:1346–1353
Assimes TL, Suissa S (2009) Age at incident treatment of hypertension and risk of cancer: a population study. Cancer Cause Control 20:1811–1820
Braun S, Bitton-Worms K, LeRoith D (2011) The link between the metabolic syndrome and cancer. Int J Biol Sci 7:1003–1015
O’Doherty MG, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC (2012) A prospective cohort study of obesity and risk of oesophageal and gastric adenocarcinoma in the NIH-AARP Diet and Health Study. Gut 61:1261–1268
Paz G, Lim EL, Wong ML, Licinio J (2011) Associations between adipokines and obesity-related cancer. Front Biosci Landmark 16:1634–1650
Zhang ZF, Kurtz RC, Sun M et al (1996) Adenocarcinomas of the esophagus and gastric cardia: medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev 5:761–768
Hohn AR, Dwyer KM, Dwyer JH (1994) Blood pressure in youth from four ethnic groups: the Pasadena Prevention Project. J Pediatr 125:368–373
Meyer P (1987) Increased intracellular calcium: from hypertension to cancer. J Hypertens 5:S3–S4
Tian T, Zhang LQ, Ma XH, Zhou JN, Shen J (2012) Diabetes mellitus and incidence and mortality of gastric cancer: a meta-analysis. Exp Clin Endocrinol Diabetes 120:217–223
Acknowledgments
The authors wish to acknowledge the services of CONOR, the contributing research centers delivering data to CONOR and all the study participants. The following cohorts from CONOR were used in the analysis: Tromsø IV and V, TROFINN, HUNT2, Oslo I, HUBRO, The Immigrant Study, MoRo, Oslo II, OPPHED, and HUSK. The Nord-Trøndelag Health Study (The HUNT Study) is performed through collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health. The study has used data from the Cancer Registry of Norway. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Cancer Registry of Norway is intended nor should be inferred.
Author contributions
All authors designed and conceptualized the article. Eivind Ness-Jensen and Kristian Hveem provided provision of study materials. Eivind Ness-Jensen, Kristian Hveem and Yunxia Lu collected and assembled data. Yulan Lin, Eivind Ness-Jensen, Jesper Lagergren and Yunxia Lu analyzed and interpreted the data. All authors wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
This work was supported by the Faculty Funds for Partial Financing of New Doctoral Students from Karolinska Institutet (12059012/KID-medel 2010); the Swedish Research Council (SIMSAM); and the Swedish Society of Medicine.
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, Y., Ness-Jensen, E., Hveem, K. et al. Metabolic syndrome and esophageal and gastric cancer. Cancer Causes Control 26, 1825–1834 (2015). https://doi.org/10.1007/s10552-015-0675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-015-0675-4