Abstract
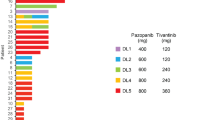
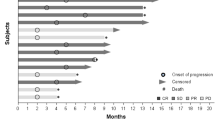
This phase 1 study evaluated the safety and tolerability of antiangiogenic therapy using vandetanib and metronomic cyclophosphamide and methotrexate in metastatic breast cancer. Eligible patients had metastatic breast cancer with 0–4 prior chemotherapy regimens. All received cyclophosphamide 50 mg daily, methotrexate 2.5 mg days 1–2 weekly, and vandetanib daily in 3 dose-escalation cohorts: 100 mg (C1), 200 mg (C2), and 300 mg (C3). The primary endpoint was safety and tolerability; secondary endpoints included response rate and evaluation of platelet-associated proteins. Twenty three patients were treated and evaluable for toxicity. Common mild toxicities included nausea, vomiting, LFTs abnormalities, fatigue, and rash. Three episodes of dose-limiting toxicity occurred in C3. In all cohorts, 1/3 of patients required vandetanib dose reduction, and 22 % ended therapy for toxicity. Of the 20 response-evaluable patients, 10 % demonstrated partial response and 15 % stable disease ≥24 weeks. Proteomic analyses demonstrated changes in platelet content of angiogenesis regulators, including vascular endothelial growth factor and platelet factor 4, with exposure to therapy. This regimen was tolerable at a maximum vandetanib dose of 200 mg; modest clinical activity was observed in this heavily pretreated population. Changes in the platelet proteome may serve as pharmacodynamic markers of angiogenesis inhibition. Metronomic chemotherapy is an attractive partner with biologics and deserves further study in metastatic breast cancer.



Similar content being viewed by others
References
Gasparini G (1999) Angiogenesis in breast cancer: role in biology, tumor progression, and prognosis. In: Bowcock A (ed) Breast cancer: molecular genetics, pathogenesis and therapeutics. Humana Press, Totowa, pp 347–371
Sledge GW Jr (2002) Vascular endothelial growth factor in breast cancer: biologic and therapeutic aspects. Semin Oncol 29:104–110
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358:2039–2049
O’Shaughnessy J, Miles D, Gray R, Dieras V, Perez E, Zon R, Cortes J, Zhou X, Phan S, Miller K (2010) A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC). J Clin Oncol 28:A1005
Sandler A, Gray R, Perry MC, Brahmer J, Schiller J, Dowlati A, Lilienbaum R, Johnson D (2006) Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer N Engl J Med 355:2542–2550
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350:2335–2342
Holden SN, Eckhardt SG, Basser R, de Boer R, Rischin D, Green M, Rosenthal MA, Wheeler C, Barge A, Hurwitz HI (2005) Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol 16:1391–1397
Herbst RS, Sun Y, Eberhardt WE, Germonpre P, Saijo N, Zhou C, Wang J, Li L, Kabbinavar F, Ichinose Y, Qin S, Zhang L, Biesma B, Heymach JV, Langmuir P, Kennedy SJ, Tada H, Johnson BE (2010) Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol 11:619–626
Heymach JV, Paz-Ares L, De Braud F, Sebastian M, Stewart DJ, Eberhardt WE, Ranade AA, Cohen G, Trigo JM, Sandler AB, Bonomi PD, Herbst RS, Krebs AD, Vasselli J, Johnson BE (2008) Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 26:5407–5415
Wells S, Gosnell J, Gagel R, Moley J, Pfister D, Sosa J, Skinner M, Krebs A, Hou J, Schlumberger M (2007) Vandetanib in metastatic hereditary medullary thyroid cancer: follow-up results of an open-label phase II trial. J Clin Oncol 25:A6018
Wells S, Robinson B, Gagel R, Dralle H, Fagin J, Santoro M, Baudin E, Vasselli J, Read J, Schlumberger M (2010) Vandetanib (VAN) in locally advanced or metastatic medullary thyroid cancer (MTC): a randomized, double-blind phase III trial (ZETA). J Clin Oncol 28:A5503
Miller KD, Trigo JM, Wheeler C, Barge A, Rowbottom J, Sledge G, Baselga J (2005) A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res 11:3369–3376
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4:423–436
Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J (2000) Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res 60:1878–1886
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS (2000) Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest 105:R15–R24
Pietras K, Hanahan D (2005) A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol 23:939–952
Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C Orlando L, Cinieri S, de BF, Viale G, Goldhirsch A (2002) Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 13:73–80
Orlando L, Cardillo A, Rocca A, Balduzzi A, Ghisini R, Peruzzotti G, Goldhirsch A, D’Alessandro C, Cinieri S, Preda L, Colleoni M (2006) Prolonged clinical benefit with metronomic chemotherapy in patients with metastatic breast cancer. Anticancer Drugs 17:961–967
Wong NS, Buckman RA, Clemons M, Verma S, Dent S, Trudeau ME, Roche K, Ebos J, Kerbel R, Deboer GE, Sutherland DJ, Emmenegger U, Slingerland J, Gardner S, Pritchard KI (2010) Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol 28:723–730
Burstein HJ, Spigel D, Kindsvogel K, Parker LM, Bunnell CA, Partridge AH, Come SE, Ryan PD, Gelman R, Winer EP (2005) Metronomic chemotherapy with and without bevacizumab for advanced breast cancer: a randomized phase II study. Breast Cancer Res Treat 94 A4
Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, Groshen S, Swenson S, Markland F, Gandara D, Scudder S, Morgan R, Chen H, Lenz HJ, Oza AM (2008) Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol 26:76–82
Garcia-Saenz JA, Martin M, Calles A, Bueno C, Rodriguez L, Bobokova J, Custodio A, Casado A, Diaz-Rubio E (2008) Bevacizumab in combination with metronomic chemotherapy in patients with anthracycline- and taxane-refractory breast cancer. J Chemother 20:632–639
Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, Shaked Y, Mancuso P, Goldhirsch A, Rocca A, Pietri E, Colleoni M (2008) Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol 26:4899–4905
Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E (2008) Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol 10:583–586
Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL (2008) Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111:1227–1233
Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almog N, Kieran MW, Folkman J (2009) Platelets actively sequester angiogenesis regulators. Blood 113:2835–2842
Cervi D, Yip TT, Bhattacharya N, Podust VN, Peterson J, Abou-Slaybi A, Naumov GN, Bender E, Almog N, Italiano JE Jr, Folkman J, Klement GL (2008) Platelet-associated PF-4 as a biomarker of early tumor growth. Blood 111:1201–1207
Ma L, Perini R, McKnight W, Dicay M, Klein A, Hollenberg MD, Wallace JL (2005) Proteinase-activated receptors 1 and 4 counter-regulate endostatin and VEGF release from human platelets. Proc Natl Acad Sci USA 102:216–220
Sierko E, Wojtukiewicz MZ (2004) Platelets and angiogenesis in malignancy. Semin Thromb Hemost 30:95–108
Peterson JE, Zurakowski D, Italiano JE Jr, Michel LV, Fox L, Klement GL, Folkman J (2010) Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol 85:487–493
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8:579–591
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Mayer EL, Dallabrida SM, Rupnick MA, Redline WM, Hannagan K, Ismail NS, Burstein HJ, Beckman JA (2011) Contrary effects of the receptor tyrosine kinase inhibitor vandetanib on constitutive and flow-stimulated nitric oxide elaboration in humans. Hypertension 58:85–92
Park Y, Downing SR, Kim D, Hahn WC, Li C, Kantoff PW, Wei LJ (2007) Simultaneous and exact interval estimates for the contrast of two groups based on an extremely high dimensional variable: application to mass spec data. Bioinformatics 23:1451–1458
Downing S, Klement G (2010) Isolation and proteomic analysis of platelets by SELDI-ToF MS. In: Clarke C, McCarthy D (eds) Methods in molecular biology: SELDI-ToF-MS: applications and protocols. Humana Press, Totowa
Bocci G, Man S, Green SK, Francia G, Ebos JM, du Manoir JM, Weinerman A, Emmenegger U, Ma L, Thorpe P, Davidoff A, Huber J, Hicklin DJ, Kerbel RS (2004) Increased plasma vascular endothelial growth factor (VEGF) as a surrogate marker for optimal therapeutic dosing of VEGF receptor-2 monoclonal antibodies. Cancer Res 64:6616–6625
Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD (2008) Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol 26:1810–1816
Ivy SP, Wick JY, Kaufman BM (2009) An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol 6:569–579
Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM Jr, Wiesenfeld M, Flynn PJ, Fitch TR, Perez EA (2009) Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol 27:11–15
Bianchi G, Loibl S, Zamagni C, Salvagni S, Raab G, Siena S, Laferriere N, Pena C, Lathia C, Bergamini L, Gianni L (2009) Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs 20:616–624
Ratain MJ, Eckhardt SG (2004) Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol 22:4442–4445
Baselga J, Segalla JG, Roche H, Del Giglio A, Pinczowski H, Ciruelos EM, Filho SC, Gomez P, Van Eyll B, Bermejo B, Llombart A, Garicochea B, Duran MA, Hoff PM, Espie M, de Moraes AA, Ribeiro RA, Mathias C, Gil Gil M, Ojeda B, Morales J, Kwon Ro S, Li S, Costa F (2012) Sorafenib in combination with capecitabine: an oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol 30:1484–1491
Gradishar W, Kaklamani V, Prasad Sahoo T, Lokanatha D, Raina V, Bondarde S, Jain M, Schwartzberg L (2009) A double-blind, randomized, placebo-controlled, phase 2b study evaluating the efficacy and safety of sorafenib (SOR) in combination with paclitaxel (PAC) as a first-line therapy in patients (pts) with locally recurrent or metastatic breast cancer (BC). Cancer Res 69:A44
Rugo HS, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, Liau KF, Kim S, Bycott P, Rosbrook B, Bair AH, Soulieres D (2011) Randomized, placebo-controlled, double-blind, phase II. Study of axitinib plus docetaxel versus docetaxel plus. Placebo in patients with metastatic breast cancer. J Clin Oncol 29:2459–2465
Boer K, Lang I, Llombart-Cussac A, Andreasson I, Vivanco GL, Sanders N, Pover GM, Murray E (2012) Vandetanib with docetaxel as second-line treatment for advanced breast cancer: a double-blind, placebo-controlled, randomized phase II study. Invest New Drugs 30:681–687
Mayer EL, Dhakil S, Patel T, Sundaram S, Fabian C, Kozloff M, Qamar R, Volterra F, Parmar H, Samant M, Burstein HJ (2010) SABRE-B: an evaluation of paclitaxel and bevacizumab with or without sunitinib as first-line treatment of metastatic breast cancer. Ann Oncol 21:2370–2376
Rini BI, Garcia JA, Cooney MM, Elson P, Tyler A, Beatty K, Bokar J, Mekhail T, Bukowski RM, Budd GT, Triozzi P, Borden E, Ivy P, Chen HX, Dolwati A, Dreicer R (2009) A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res 15:6277–6283
Brown AP, Citrin DE, Camphausen KA (2008) Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis Rev 27:415–434
Hanrahan EO, Ryan AJ, Mann H, Kennedy SJ, Langmuir P, Natale RB, Herbst RS, Johnson BE, Heymach JV (2009) Baseline vascular endothelial growth factor concentration as a potential predictive marker of benefit from vandetanib in non-small cell lung cancer. Clin Cancer Res 15:3600–3609
Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV (2007) Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res 13:2643–2650
Mancuso P, Colleoni M, Calleri A, Orlando L, Maisonneuve P, Pruneri G, Agliano A, Goldhirsch A, Shaked Y, Kerbel RS, Bertolini F (2006) Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood 108:452–459
Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, Flockhart DA, Hancock B, Davidson N, Gralow J, Dickler M, Perez EA, Cobleigh M, Shenkier T, Edgerton S, Miller KD (2008) Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 26:4672–4678
Willis S, Miller K, Young B, Perou C, Hu Z, Sparano J, Gray R, Sledge G, Davidson N, Leyland-Jones B (2012) Association of a compact 13-gene VEGF signature with OS in E2100. J Clin Oncol 30:A1027
Acknowledgments
This research was supported by a grant from the Investigator-Sponsored Study Program of AstraZeneca, Wilmington, DE
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
www.clinicaltrials.gov identification number: NCT00496665.
Rights and permissions
About this article
Cite this article
Mayer, E.L., Isakoff, S.J., Klement, G. et al. Combination antiangiogenic therapy in advanced breast cancer: a phase 1 trial of vandetanib, a VEGFR inhibitor, and metronomic chemotherapy, with correlative platelet proteomics. Breast Cancer Res Treat 136, 169–178 (2012). https://doi.org/10.1007/s10549-012-2256-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2256-5