Abstract
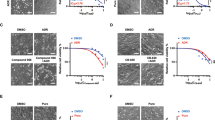
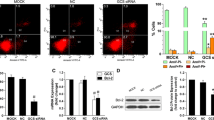
Drug resistance in breast cancer remains a major cause for the failure of chemotherapy. Glucosylceramide synthase (GCS) plays an important role in multidrug resistance (MDR) in breast cancer. P-glycoprotein (P-gp) also confers a cross-resistance of many unrelated drugs. In this study, we studied the MDR effect and potential mechanisms of breast cancer after constructing permanent breast cancer cell lines with GCS knockout by using recombinant vectors targeting GCS (pSUPER-GCSshRNAs). The GCSshRNA stably transfected cells were successfully established and significant lower levels of GCS mRNA and protein expression were confirmed. In in vitro experiments, the GCSshRNA stably transfected cells showed a significantly reduced level of MDR1 and P-gp expression and decreased drug efflux ability. Reduced level of GCS expression conveyed a significant reversal of drug resistance by MTT assay and increased caspase-3 activity. In in vivo experiments by using nude mice with xenograft tumors, a significant inhibition of tumor growth was observed after comparing with the control group. Furthermore, enhanced response of chemotherapy was acquired by reduced expression of GCS as well as MDR1 in vivo. In conclusion, GCSshRNA could efficiently suppress GCS and MDR1 expression in vitro and in vivo and these findings may be used as one of the methods to reverse MDR in breast cancer.








Similar content being viewed by others
Abbreviations
- GCS:
-
Glucosylceramide synthase
- MDR:
-
Multidrug resistance
- P-gp:
-
P-glycoprotein
- RNAi:
-
RNA interference
- siRNA:
-
Small interfering RNA
- shRNA:
-
Short hairpin RNA
References
Takara K, Sakaeda T, Okumura K (2006) An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr Pharm Des 12:273–286
Kohyama-Koganeya A, Sasamura T, Oshima E et al (2004) Drosophila glucosylceramide synthase: a negative regulator of cell death mediated by proapoptotic factors. J Biol Chem 279:35995–36002
Lavie Y, Cao H, Bursten SL et al (1996) Accumulation of gulcosylceramide in multidrug-resistant cancer cells. J Biol Chem 271:19530–19536
Liu YY, Han TY, Giuliano AE et al (1999) Expression of glucosylceramide synthase, converting ceramide to glucosylceramide, confers adriamycin resistance in human breast cancer cells. J Biol Chem 274:1140–1146
Cai Z, Bettaieb A, Mahdani NE et al (1997) Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF-7 cells to tumor necrosis factor-α-mediated cytotoxicity. J Biol Chem 272:6918–6926
Volm M (1998) Multidrug resistance and its reversal. Anticancer Res 18:2905–2909
Zrieki A, Farinotti R, Buyse M (2008) Cyclooxygenase inhibitors down regulate P-glycoprotein in human colorectal Caco-2 cell line. Pharm Res 25:1991–2001
Kitagawa S (2006) Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol Pharm Bull 29:1–6
Saeki T, Tsuruo T, Sato W et al (2005) Drug resistance in chemotherapy for breast cancer. Cancer Chemother Pharmacol 56:84–89
Holland ML, Panetta JA, Hoskins JM et al (2006) The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem Pharmacol 71:1146–1154
Mungall BA, Schopman NC, Lambeth LS et al (2008) Inhibition of Henipavirus infection by RNA interference. Antiviral Res 80:324–331
Chu CY, Rana TM (2008) Potent RNAi by short RNA triggers. RNA 14:1714–1719
Burkhardt BR, Lyle R, Qian K et al (2006) Efficient delivery of siRNA into cytokine-stimulated insulinoma cells silences Fas expression and inhibits Fas-mediated apoptosis. FEBS Lett 580:553–560
Delgado R, Regueiro BJ (2005) The future of HIV infection: gene therapy and RNA interference. Enferm Infecc Microbiol Clin 23:76–83
Devi GR (2006) siRNA-based approaches in cancer therapy. Cancer Gene Ther 13:819–829
Amarzguioui M, Rossi JJ, Kim D (2005) Approaches for chemically synthesized siRNA and vector-mediated RNAi. FEBS Lett 579:5974–5981
Kumar P, Lee SK, Shankar P et al (2006) A single siRNA suppresses fatal encephalitis induced by two different flaviviruses. PLoS Med 3:e96
Stein U, Walther W, Stege A et al (2008) Complete in vivo reversal of the multidrug resistance phenotype by jet-injection of anti-MDR1 short hairpin RNA-encoding plasmid DNA. Mol Ther 16:178–186
Matters GL, Harms JF, McGovern CO et al (2009) Growth of human pancreatic cancer is inhibited by down-regulation of gastrin gene expression. Pancreas 38:151–161
Chen Y, Chen H, Hoffmann A et al (2006) Adenovirus-mediated small-interference RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension 47:145–146
Brezinsky SC, Chiang GG, Szilvasi A et al (2003) A simple method for enriching populations of transfected CHO cells for cells of higher specific productivity. J Immunol Methods 277:141–155
Volm M, Kästel M, Mattern J et al (1993) Expression of resistance factors (P-glycoprotein, glutathione S-transferase-pi, and topoisomerase II) and their interrelationship to proto-oncogene products in renal cell carcinomas. Cancer Cell 71:3981–3987
Marzolini C, Paus E, Buclin T et al (2004) Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol 75:13–33
Kok JW, Veldman RJ, Klappe K et al (2000) Differential expression of sphingolipids in MRP1 overexpressing HT29 cells. Int J Cancer 87:172–178
Nicholson KM, Quinn DM, Kellett GL et al (1999) Preferential killing of multidrug-resistant KB cells by inhibitors of glucosylceramide synthase. Br J Cancer 81:423–430
Gouazé-Andersson VYJ, Kreitenberg AJ et al (2007) Ceramide and glucosylceramide upregulate expression of the multidrug resistance gene MDR1 in cancer cells. Biochim Biophys Acta 1771:1407–1417
Shabbits JA, Mayer LD (2002) P-glycoprotein modulates ceramide-mediated sensitivity of human breast cancer cells to tubulin-binding anticancer drugs. Mol Cancer Ther 1:205–213
Liu YY, Han TY, Giuliano AE et al (2000) Uncoupling ceramide glycosylation by transfection of glucosylceramide synthase antisense reverses adriamycin resistance. J Biol Chem 275:138–143
Bentires-Alj M, Barbu V, Fillet M et al (2003) NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene 22:90–97
Kanto T, Kalinski P, Hunter OC et al (2001) Ceramide mediates tumor-induced dendritic cell apoptosis. J Immunol 167:3773–3784
Hara S, Nakashima S, Kiyono T et al (2004) Ceramide triggers caspase activation during gamma-radiation-induced apoptosis of human glioma cells lacking functional p53. Oncol Rep 12:119–123
Deng W, Li R, Guerrera M et al (2002) Transfection of glucosylceramide synthase antisense inhibits mouse melanoma formation. Glycobiology 12:145–152
Guerrera M, Ladisch S (2003) N-butyldeoxynojirimycin inhibits murine melanoma cell ganglioside metabolism and delays tumor onset. Cancer Lett 201:31–40
Acknowledgments
This work was supported in part by National Nature Science Foundation of China (No. 30300124); Nature Science Foundation of Shandong Province of China (No. Y2007C112, Y2007C15).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Dr. Yanlin Sun and Dr. Gengyin Zhou contributed equally to this study.
Rights and permissions
About this article
Cite this article
Sun, Y., Zhang, T., Gao, P. et al. Targeting glucosylceramide synthase downregulates expression of the multidrug resistance gene MDR1 and sensitizes breast carcinoma cells to anticancer drugs. Breast Cancer Res Treat 121, 591–599 (2010). https://doi.org/10.1007/s10549-009-0513-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0513-z