Abstract
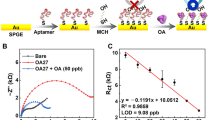
Okadaic acid (OA) is a marine toxin ingested by shellfish. In this work, a simple, sensitive and label-free gap-based electrical competitive bioassay has been developed for this biotoxin detection. The gap-electrical biosensor is constructed by modifying interdigitated microelectrodes with gold nanoparticles (AuNPs) and using the self-catalytic growth of AuNPs as conductive bridges. In this development, the AuNPs growth is realized in the solution of glucose and chloroauric acid, with glucose oxidation used as the catalysis for growth of the AuNPs. The catalytic reaction product H2O2 in turn reduces chloroauric acid to make the AuNPs grow. The conductance signal amplification is directly determined by the growth efficiency of AuNPs and closely related to the catalytic activity of AuNPs upon their interaction with OA molecule and OA aptamer. In the absence of OA molecule, the OA aptamer can absorb onto the surfaces of AuNPs due to electrostatic interaction, and the catalytically active sites of AuNPs are fully blocked. Thus the AuNPs growth would not happen. In contrast, the presence of OA molecule can hinder the interaction of OA aptamer and AuNPs. Then the AuNPs sites are exposed and the catalytic growth induces the conductance signal change. The results demonstrated that developed biosensor was able to specifically respond to OA ranging from 5 ppb to 80 ppb, providing limit of detection of 1 ppb. The strategy is confirmed to be effective for OA detection, which indicates the label-free OA biosensor has great potential to offer promising alternatives to the traditional analytical and immunological methods for OA detection.







Similar content being viewed by others
References
T.S.D. Brown, B.F. Johnson, Nucleation and growth of nano-gold colloidal lattices. Chem. Commun. 11, 1007–1008 (1997)
M. Campàs et al., Enzymatic recycling-based amperometric immunosensor for the ultrasensitive detection of okadaic acid in shellfish. Biosens. Bioelectron. 24(4), 716–722 (2008)
S. Eissa et al., Selection and identification of DNA aptamers against okadaic acid for Biosensing application. Anal. Chem. 85(24), 11794–11801 (2013)
A.D. Ellington, J.W. Szostak, In vitro selection of RNA molecules that bind specific ligands. Nature 346(6287), 818–822 (1990)
K.C. Grabar et al., Preparation and characterization of Au colloid monolayers. Anal. Chem. 67(4), 735–743 (1995)
A. Hayat, L. Barthelmebs, J.-L. Marty, Enzyme-linked immunosensor based on super paramagnetic nanobeads for easy and rapid detection of okadaic acid. Anal. Chim. Acta 690(2), 248–252 (2011a)
A. Hayat et al., An electrochemical immunosensor based on covalent immobilization of okadaic acid onto screen printed carbon electrode via diazotization-coupling reaction. Talanta 85(1), 513–518 (2011b)
A. Hayat, L. Barthelmebs, J.-L. Marty, A simple colorimetric enzymatic-assay for okadaic acid detection based on the immobilization of protein phosphatase 2A in sol-gel. Appl. Biochem. Biotechnol. 166(1), 47–56 (2012a)
A. Hayat et al., Development of a novel label-free amperometric immunosensor for the detection of okadaic acid. Anal. Chim. Acta 724, 92–97 (2012b)
H. Huang et al., Determination of okadaic acid and Dinophysistoxin-1 in mussels by high-performance liquid chromatography–tandem mass spectrometry. Anal. Lett. 47(12), 1987–1994 (2014)
N. Impens, P. Van Der Voort, E. Vansant, Silylation of micro-, meso-and non-porous oxides: A review. Microporous Mesoporous Mater. 28(2), 217–232 (1999)
M.P. Kreuzer, C.K. O'Sulliva, G.G. Guilbault, Development of an ultrasensitive immunoassay for rapid measurement of okadaic acid and its isomers. Anal. Chem. 71(19), 4198–4202 (1999)
S.-Y. Lu et al., Production of monoclonal antibody and application in indirect competitive ELISA for detecting okadaic acid and dinophytoxin-1 in seafood. Environ. Sci. Pollut. Res. 19(7), 2619–2626 (2012)
S.L. Morton, R.T. Donald, Determination of okadaic acid content of dinoflagellate cells: A comparison of the HPLC-fluorescent method and two monoclonal antibody ELISA test kits. Toxicon 34(8), 947–954 (1996)
B. Prieto-Simón et al., High-sensitive flow-based kinetic exclusion assay for okadaic acid assessment in shellfish samples. Biosens. Bioelectron. 25(6), 1395–1401 (2010)
A. Sassolas et al., Development of an efficient protein phosphatase-based colorimetric test for okadaic acid detection. Anal. Chim. Acta 702(2), 262–268 (2011)
A. Sassolas et al., Detection of the marine toxin okadaic acid: Assessing seafood safety. Talanta 105, 306–316 (2013a)
A. Sassolas et al., Improvement of the efficiency and simplification of ELISA tests for rapid and ultrasensitive detection of okadaic acid in shellfish. Food Control 30(1), 144–149 (2013b)
H. Shiigi et al., Label-free electronic detection of DNA-hybridization on Nanogapped gold particle film. J. Am. Chem. Soc. 127(10), 3280–3281 (2005)
A. Takai et al., Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 284, 539–544 (1992)
A.X.J. Tang et al., Immunosensor for okadaic acid using quartz crystal microbalance. Anal. Chim. Acta 471(1), 33–40 (2002)
S. Tokonami, H. Shiigi, T. Nagaoka, Open bridge-structured gold nanoparticle Array for label-free DNA detection. Anal. Chem. 80(21), 8071–8075 (2008)
C. Tuerk, L. Gold, Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249(4968), 505–510 (1990)
R.D. Vaughan et al., Piezoelectric immunosensors for environmental monitoring. Int. J. Environ. Anal. Chem. 83(7–8), 555–571 (2003)
M.M. Vdovenko et al., Determination of okadaic acid in shellfish by using a novel chemiluminescent enzyme-linked immunosorbent assay method. Talanta 116, 343–346 (2013)
G. Volpe et al., A bienzyme electrochemical probe for flow injection analysis of okadaic acid based on protein phosphatase-2A inhibition: An optimization study. Anal. Biochem. 385(1), 50–56 (2009)
R. Wojcieszak et al., Selective oxidation of glucose to glucuronic acid by cesium-promoted gold nanoparticle catalyst. J. Mol. Catal. A Chem. 422, 35–42 (2016)
J. Zhang et al., Self-catalytic growth of unmodified gold nanoparticles as conductive bridges mediated gap-electrical signal transduction for DNA hybridization detection. Anal. Chem. 86(2), 1178–1185 (2013)
Acknowledgements
This work was supported by Major International Cooperation Project of Natural Science Foundation of China (No. 61320106002), Marine Public Welfare Project of China (No.201305010), and Natural Science Foundation of China (No. 31228008, 81501553).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, Y., Wan, Z., Zhong, L. et al. Label-free okadaic acid detection using growth of gold nanoparticles in sensor gaps as a conductive tag. Biomed Microdevices 19, 33 (2017). https://doi.org/10.1007/s10544-017-0162-7
Published:
DOI: https://doi.org/10.1007/s10544-017-0162-7