Abstract
Acetic acid bacteria are versatile organisms converting a number of carbon sources into biomolecules of industrial interest. Such properties, together with the need to limit chemical syntheses in favor of more sustainable biological processes, make acetic acid bacteria appropriate organisms for food, chemical, medical, pharmaceutical and engineering applications. At current, well-established bioprocesses by acetic acid bacteria are those derived from the oxidative pathways that lead to organic acids, ketones and sugar derivates. Whereas emerging applications include biopolymers, such as bacterial cellulose and fructans, which are getting an increasing interest for the biotechnological industry. However, considering the industrial demand of high performing bioprocesses, the production yield of metabolites obtained by acetic acid bacteria, is still not satisfying. In this paper we review the major acetic acid bacteria industrial applications, considering the current status of bioprocesses. We will also describe new biotechnological advances in order to optimize the industrial production, offering also an overview on future directions.
Similar content being viewed by others
Introduction
The acetic acid bacteria (AAB) group includes highly versatile organisms, able to produce a great variety of compounds used in food and beverages, chemical, medical, pharmaceutical and engineering fields (De Roos and De Vuyst 2018; Mamlouk and Gullo 2013).
The genus Acetobacter includes species (i.e. Acetobacter pasteurianus and A. aceti) of interest for the food industry because of the ethanol-oxidizing activity that is beneficial in vinegar production and detrimental in other fermented beverages like wine, beer and cider (Bartowsky and Henschke 2008; Sengun and Karabiyikli 2011; Wu et al. 2010).
Gluconacetobacter and Komagataeibacter genera, on the other hand, comprise highly versatile species, having a great variety of metabolic abilities including acetic acid and gluconic acid production, nitrogen fixation and bacterial cellulose production. For instance K. europaeus, which is the main species involved in industrial vinegar production, K. xylinus, which is designated as a model organism of bacterial cellulose (BC) synthesis and Ga. diazotrophicus that includes strains of interest for their endophytic activity (Mamlouk and Gullo 2013).
Within Gluconobacter genus, G. oxydans plays a leading biotechnological role because of its importance for the industrial production of gluconic acid, dihydroxyacetone, Vitamin C (l-ascorbic acid) precursors and miglitol (Adachi et al. 2003; De Vero et al. 2010; Sainz et al. 2016; Dikshit and Moholkar 2018).
Recently, species of Gluconobacter, Gluconacetobacter, Komagataeibacter, Kozakia and Neoasaia genera have been investigated for their ability to produce fructans such as levan-type exopolysaccharides (LT-EPS). Such compounds are getting increasing attention due to their possible use in medical, pharmaceutical and foods fields (Jakob et al. 2012a; Brandt et al. 2016).
This review focuses on the metabolic potential of AAB taking into account their versatility in the bioconversion of carbon substrates into valuable bioproducts, such as organic acid, ketones and exopolysaccharides (Table 1). Is it known that other metabolites, not discussed in this paper, are of industrial interest, however here, we will focus on acetic acid, gluconic acid, dihydroxyacetone, 2-keto-l-gulonic acid, BC and fructans, as representative of both consolidated and emerging applications of AAB (Table 2).
Main bioconversions having industrial impact
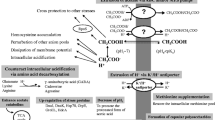
The oxidation of carbon sources by AAB is responsible for the production of a number of industrially relevant compounds. In such processes, sugars, alcohols and sugar alcohols are incompletely oxidized to the corresponding products, which are accumulated in large amounts in the medium. These pathways, the so-called oxidative fermentations, involve a set of membrane-bound dehydrogenases, located in the periplasmic region of the cell membrane. Considering their primary structure, membrane-bound dehydrogenases of AAB (also called primary dehydrogenases) can be divided into five groups: quinoprotein-cytochrome complex, molybdoprotein-cytochrome complex, flavoprotein-cytochrome complex, membrane-bound quinoprotein, and other proteins (Adachi and Yakushi 2016). They first extract the electrons from the substrates; then transfer them to the terminal oxidase via ubiquinone, which generates proton-motive force for ATP generation (Matsushita et al. 1994). The respiratory chain is involved only in the partial oxidation, but not in the complete oxidation of these substrates to CO2 and H2O. The assimilation pathways of partially oxidized products (for instance those related to acetic acid, ketogluconates or dihydroxyacetone), operating in the cytoplasm, generally, are active only in the late growth or in the stationary phase (Toyama et al. 2005). Historically, AAB have been known for their ability to produce acetic acid by oxidation of ethanol. This reaction can occur either on the periplasmic side of the cell membrane or in cytosol. On the periplasmic side, ethanol is first oxidized to acetaldehyde by a membrane bound pyrroloquinoline quinone-dependent alcohol dehydrogenase (PQQ-ADH) and then acetaldehyde is further oxidized to acetic acid by aldehyde dehydrogenase (ALDH), which belong to the molybdoprotein-cytochrome complex. In the cytosol, the pathway is identical, but the bioconversion is operated by both a NAD-dependent ADH [EC 1.1.1.1] and ALDH [EC 1.2.1.3]. After ethanol depletion, the accumulated acetic acid is used by cells via acetyl CoA synthase and phosphoenolpyruvate carboxylase.
The high versatility of AAB emerges when we consider sugars metabolism, which can generate a number of oxidation products and exopolysaccharides like BC and fructans.
Glucose oxidation, as mainly studied in G. oxydans, leads to the synthesis of glucono-δ-lactone acid in a reaction catalysed by the membrane-bound pyrroloquinolinequinone-dependent gluconate dehydrogenase (PQQ-GDH). Glucono-δ-lactone is stable in acid conditions, but it can spontaneously hydrolyse to gluconic acid under neutral and alkaline conditions or can be converted to gluconic acid by a membrane-bound gluconolactonase (Ameyama et al. 1981; Matsushita et al. 1994).
Gluconic acid can be further oxidized to 2-keto-d-gluconate by the flavoprotein gluconate dehydrogenase (FAD-GADH) or to 5-keto-d-gluconate by the PQQ-glycerol dehydrogenase (PQQ-GLDH), a polyol-dehydrogenase (also known as d-sorbitol dehydrogenase (SLDH)) which have a broad substrate specificity (Saichana et al. 2015). 2-keto-d-gluconate is oxidized to 2,5-diketo-d-gluconate acid by the flavoprotein 2-keto-d-gluconate dehydrogenase (FAD-2KGADH) [EC 1.1.99.4]. Both, 5-keto-d-gluconate and 2,5-diketo-d-gluconate, are intermediate products of the synthesis of 2-keto-l-gulonic acid, a precursor in the vitamin C process. The substrates for the production of 2-keto-l-gulonic acid can be glucose, d-sorbitol or l-sorbose, respectively. When d-glucose is used as substrate, 2-keto-l-gulonic acid is formed via 2,5-diketo-d-gluconate, as mentioned before (Sonoyama et al. 1982). In the case of d-sorbitol as substrate, it is first oxidized to l-sorbose (PQQ-GLDH) and then to l-sorbosone by the flavoprotein l-sorbose dehydrogenase (FAD-SDH) (Sugisawa et al. 1991). If l-sorbose is utilized as substrate, 2-keto-l-gulonic acid is produced via l-sorbosone (Hoshino et al. 1990). Finally, l-sorbosone is oxidized to 2-keto-l-gulonic by l-sorbosone dehydrogenase (SNDH), which cofactor remain unknown (Adachi and Yakushi 2016).
In AAB, sugars can be also used as monomeric units for the synthesis of exopolysaccharides chains, such as BC and fructans. BC is produced from activated glucose (UDP-glucose) as starting material, thanks to cellulose synthase complex (CS) activity that forms 1,4-β-glucan chains. The CS is constituted by different subunits that work in a concerted way. The polymerization mechanism of 1,4-β-glucan chains to form crystalline structure is characterized by events series that occur in that so called terminal complex (TC), outside the cell. 16 CSs are localized in each TC that form 16 glucan chains. The first polymerization event occurs between glucan chains in order to form a protofibrils, thanks to the establishment of hydrogen bonds and Van der Waals forces. Protofibrils from adjacent TC polymerize to form microfibrils and finally the wound ribbon (Ross et al. 1991). Among fructans, LT-EPS are synthesized by the extracellular enzyme levansucrase (or sucrose 6-fructosyltransferase, which catalyses the transfer of d-fructosyl residues from sucrose to a growing fructan chain by trans-fructosylation (Donot et al. 2012; Han and Clarke 1990). Once sucrose is completely depleted, levansucrase cleaves the ß(2–6) linkages of the newly-formed levan chain, causing the consecutive release of the terminal fructose units until a branching point is reached (Chambert et al. 1974; Méndez-Lorenzo et al. 2015).
Acetic acid
Acetic acid has two major areas of production: the first one is food-grade vinegar and the second one chemically synthesized acetic acid (mainly produced from petrochemical sources, such as carbonylation of methanol and oxidation of n-butane), a commodity that has become an important feedstock for the chemical industry (Vidra and Németh 2018). The market for acetic acid is growing at ca. 5% per year and estimated to 16,155 metric tons by 2020. Over 65% of global production of pure acetic acid is obtained by chemical synthesis and not biologically. The chemical process via methanol carbonylation, ensures high starting acetic acid concentrations (35–45%), high yield (about 99% from methanol) and low production costs. While the main disadvantage of the biological production, is the cost to recover low concentrations of acetic acid (4–12%) from fermentation broths (Murali et al. 2017; Rogers et al. 2006).
The acetic acid obtained by microbial oxidative conversion of ethanol-containing substrates, is worldwide referred to vinegar. Although in vinegar industry the bioconversion of ethanol in acetic acid is defined highly efficient (about 90–95% of the stoichiometric yield) (Jiménez-Hornero et al. 2009), many evidences underline the need of further optimization. Considering the applied aspects of the fermentation process, it is remarkable to emphasize that a main difference between vinegar production and most other fermented foods and beverages (e.g. sourdough for bread production, dairy products, sausages wine and beer) is the kind of microbial culture used. In particular, to obtain vinegar, fermentations are developed by mixed microbial cultures, without using selected starter cultures (SSCs), at both small and large scale (Gullo et al. 2014; Hidalgo et al. 2010; Mas et al. 2014). Practically, heterogeneous AAB cultures are propagated by back-slopping procedure and reused to start up subsequent fermentation cycles. The selective pressure due to acetic acid, allows keeping relatively stable the AAB population. This procedure ensures low production costs, high performance and does not require specialized expertise to perform the fermentation. However, to minimize risks of fermentation breakdown and/or undesired metabolites, the introduction of SSCs is considered an appropriate technological tool for vinegar industry (Ndoye et al. 2006; Gullo and Giudici 2008).
Regarding the design and application of SSCs for submerged fermentation, first Sokollek and Hammes (1997) proved the importance of specific media to reach high cell yields and to revitalize the culture after storing, preserving the viability of industrial cultures. SSCc were also applied in static system characterized by a long fermentation time (acetic acid produced about 6% v/v). In this case a main characteristic is the attitude of the strain to persist along scaling up in not or less controlled processes (Giudici et al. 2009; Gullo et al. 2009). More recently 90 g/L of acetic acid were achieved by a SSC produced from A. pasteurianus UMCC 1754 in commercial red wine, whereas 60 g/L were produced using the same strain to ferment cooked grape must at 20% of sugars (Gullo et al. 2016). These studies confirmed the feasibility to manage vinegar fermentations by SSCs, as well as the versatility of AAB strains to adapt to different substrates in controlled fermentations.
In vinegar fermentation process, AAB are exposed to different chemical-physical stressors such as the initial ethanol amount, the increasing concentration of acetic acid and the heat generated during acetic acid production. These factors strongly affect cell growth and consequently the final acetic acid yield. Thanks to advances in the genetic engineering, different studies have been conducted to understand tolerance mechanisms and how to improve the resistance of AAB to the above mentioned parameters. These studies are mainly focused on acetic acid resistance, which is considered the main stressor under industrial conditions. This is due to the undissociated acetic acid that once penetrates the cell membrane, disintegrates membrane transport processes and causes an increase of acidity into the cell.
Qi and co-workers (2013) obtained a high-acetic acid producing A. pasteurianus mutant (CICIM B7003-02) by UV mutagenesis under acidic stress that showed enhanced fermentation ability and higher tolerance to acetic acid compared to the wild type. Concerning the mechanisms involved in tolerance to acetic acid and temperature, a study highlighted the relevance of chaperons system. In particular, GroEL and GroES subsystem seems to be involved in those mechanisms. Indeed, an increase of both production and tolerance to acetic acid was highlighted in a mutant strain of A. pasteurianus that exhibits rpoH, a gene that regulates the expression of GroES GroEL system and other chaperons, overexpressed. Whereas a disruption of RpoH induces to a higher sensitivity to ethanol, acetic acid and temperatures compared to the wild-type (Okamoto-Kainuma et al. 2011). Matsutani and co-workers (2013), instead, improved the thermotolerance of the strain A. pasteurianus SKU1108 by repeated cultivation cycles under high-temperature conditions. The mutant strain (SKU1108-TH-3), produced 30 g/L of acetic acid at 37 °C and acquired the ability to ferment at a temperature of 3 °C higher than the wild-type strain.
Among mechanisms involved in the tolerance to acetic acid, also the systems to discharge the intracellular acetic acid play a key role. Proteomics studies on A. aceti confirmed that different proteins are induced by high acetic acid concentrations. The protein AatA, a putative ABC transporter, seems to have a relevant role in acetic acid tolerance. The disruption of this protein caused a reduction of acetic acid tolerance but not of lactic acid and propionic acid, suggesting that AatA protein functions as specific exporter of intracellular acetic acid (Mendez and Salas 2001). The described alteration of acetic acid tolerance mechanisms can also affect the genome integrity, as demonstrated in A. pasteurianus (strain AC2005). In this strain it was proved that the activation of DNA repairs mechanism induced by acetic acid, protects the DNA and resolves genomic damages. The UvrA protein, a nucleotide repair excinuclease, was found to be upregulated in the presence of high acetic acid concentration, resulting in an improvement of acetic acid fermentation (Zheng et al. 2018).
Although the controversial issues in using engineered organisms, all these findings support the evidence that mechanisms of tolerance, discussed above, are key traits in AAB selection for the SSCs design.
Gluconic acid
Gluconic acid and its salts are multifunctional bulks extensively used in industries. The global market of gluconic acid achieves USD 51.6 million in 2016, and is expected to reach USD 66.92 million by 2022 (QYResearch Reports 2017).
Gluconic acid is used by pharmaceutical industry as chelating agent of divalent metals, in hygiene because of its sequestering action in alkaline media and in building industries to prevent color variations (Singh and Kumar 2007; García-García et al. 2017). Beyond its use in pharmaceutic, gluconic acid is also exploited to improve the sensorial complexity of foods and in vinegar production, in which it contributes significantly to lower the pH (lower pKa than that acetic acid) (Giudici et al. 2016). Also, glucono-δ-lactone is widely used in the food industry as a preservative for cured meat-based sausage (Cañete-Rodríguez et al. 2016). Gluconic acid derivatives, such as 2-keto-d-gluconic acid and 5-keto-l-gluconic acid are also valuable industrial compounds. 2-keto-d-gluconic acid is used as a building blocks in chemical synthesis of heterocycles compounds (Stottmeister et al. 2005). 5-keto-d-gluconic acid is used as an antioxidant in the food industry, a reducing agent in the textile industry and a chiral compound for chemical synthesis (Herrmann et al. 2004).
Given its wide use in different industrial sectors, the enhancement of gluconic acid production aimed to maximize the yield, has been widely studied. There are two major aspects to consider in the gluconic acid production: the metabolism of the organism used and the chemical-physical parameters of the processes. Among AAB the most used strains for gluconic acid production belong to G. oxydans species. As mentioned above when G. oxydans grows on glucose, most of the glucose is oxidized in gluco-δ-lactone and, then into gluconic acid. Further oxidation of gluconic acid leads to the formation of ketogluconates such as 2-keto-d-gluconate and 5-keto-d-gluconate, which are undesirable products in the gluconic acid production process. The production ratio of gluconic acid, 2-keto-d-gluconate and 5-keto-d-gluconate is strictly strain-dependent and linked to growth conditions that influence the activity of FAD-GADH and PQQ-GLDH enzymes (Ano et al. 2011; Sainz et al. 2016; Shinagawa et al. 1983).
In order to improve the yields of these compounds, different studies have been conducted by using genetic engineering approaches. The overexpression of the genes encoding for the membrane-bound enzymes such as the PQQ-GDH, FAD-GADH and PQQ-GLDH can direct the glucose oxidation pathways to one of above mentioned compounds (gluconic acid, 2-keto-d-gluconate and 5-keto-d-gluconate). Shi et al. (2014), for example, achieved an increase of two fold in the 2-keto-d-gluconate productivity by overexpressing the ga2dh gene, codifying for FAD-GADH. Merfort et al. (2006), on the other hand, obtained an increase of both gluconic acid and 5-keto-d-gluconate by equipping the strain G. oxydans MF1 with a plasmid that allows the overexpression of both PQQ-GDH and PQQ-GLDH.
Some studies highlighted the role of some chemical-physical parameters, such as pH, glucose concentration, presence of calcium carbonate (CaCO3) and aeration rate, in the gluconic acid production. A high gluconic acid production was achieved keeping the pH below 3.5–4 in glucose media since ketogluconates formation is suppressed at low pH (Roehr et al. 1996). At pH higher than 5 the formation of ketoacids was favored. Another parameter in the gluconic acid and its ketoacids production is CaCO3. The presence of CaCO3 in the culture medium considerably promotes the ketoacids production by shifting the chemical equilibrium towards the acids formation, due to the low solubility of ketogluconates calcium salts.
The aeration rate also affects the production yield since O2 is a substrate for different enzymes involved in gluconic acid pathways. A recent study showed that the O2 concentration should be kept at a saturation level, included in a range of 20–30%, to achieve the highest gluconic acid production yield (García-García et al. 2017).
Dihydroxyacetone
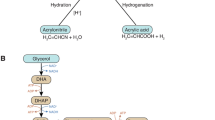
Dihydroxyacetone is a non-chiral compound used in the pharmaceutical industry as a cosmetic tanning agent and as an intermediate for the synthesis of various organic chemicals and surfactants. The global market of dihydroxyacetone is estimated in 2000 tons per year (Pagliaro et al. 2007; Lux and Siebenhofer 2013). Chemical synthesis of dihydroxyacetone through catalytic reaction of formaldehyde has low selectivity, thus industrial production is mainly operated by oxidation of glycerol via PQQ-GLDH, using G. oxydans strains (Hekmat et al. 2003; Chozhavendhan et al. 2018). Despite of many efforts in improvement of strains, it has been reported that, the microbial process of dihydroxyacetone by G. oxydans requires further optimization to maximize the yield and then the economic convenience. Main drawbacks in the microbial process are substrate (glycerol) and product (dihydroxyacetone) inhibition on bacterial growth (Macauley et al. 2001). Enhanced yield of microbial dihydroxyacetone production was obtained by immobilizing G. oxydans cells to a polyvinyl alcohol support. However, a dramatic reduction of the activity of immobilized cells, due to the loss of the electron transfer ability of the organism, was observed after only five operation batches (Wei et al. 2007).
Gätgens et al. (2007) obtained an increased dihydroxyacetone production by overexpressing sldAB protein, coding for PQQ-GLDH in G. oxydans MF1. In a study aimed to reduce product inhibition and simultaneously increase dihydroxyacetone production, the overexpression of GLDH and the interruption of adhA gene in G. oxydans allowed to obtain 134 g/L of dihydroxyacetone from 140 g/L of glycerol (Li et al. 2010). Moreover, mutants from G. oxydans with significant increased production of dihydroxyacetone, during a selection time of 25–50 transfers, have been obtained by adaptive laboratory evolution experiments using glucose as the sole carbon source (Lu et al. 2012).
Recently, dihydroxyacetone was produced from crude glycerol, a by-product of biodiesel industry. By repeated-batch experiments with four times crude glycerol feeding (10 g/L each time), a dihydroxyacetone concentration of 36 g/L (89.88% conversion rate within 96 h of fermentation) was obtained. The conversion of crude glycerol into value-added chemicals such as dihydroxyacetone, could contribute to a more economic feasible biodiesel production (Dikshit and Moholkar 2018).
2-Keto-gulonic acid as intermediate of vitamin C synthesis
Vitamin C is used in a wide range of industrial fields, such as pharmaceutical, food and beverage and cosmetic. The global revenue is expected to reach USD 1083.8 million by 2021 (QYResearch Reports 2017).
Until 1990s, vitamin C was produced by Reichstein process, a combined chemical and microbial method involving five chemical steps and one microbial fermentation by which d-sorbitol is converted in l-sorbose. More recently, different processes have been studied in order to offer eco-friendly fermentations as an alternative to the chemical synthesis. These alternative processes enhanced the role of AAB as biocatalysts for the production of vitamin C, because of their ability to synthetize 2-keto-gulonic acid by different pathways, as full described by Shinjoh and Toyama (2016). Actually, the most used species in Vitamin C production is G. oxydans (Pappenberger and Hohmann 2014).
Saito and co-workers (1997) obtained 2-keto-gulonic acid from d-sorbitol at high yield (200 g per L of d-sorbitol converted to 200 g per L of l-sorbose) by a recombinant G. oxydans strain (T-100). Both the strains and enzymes were protected by a patent of Fujisawa Pharmaceutical Co., Ltd.
The strain G. oxydans WSH-003 was proved to be efficient in the production of high amount of l-sorbose and it is industrially exploited to produce vitamin C via two-step fermentation process (Gao et al. 2014).
Enhanced production of l-sorbose (+ 25% respect to the wild strain) was obtained by engineering WSH-003 on which artificial poly (A/T) tails were added to the gene that encodes for PQQ-GLDH (Xu et al. 2014). Another study on WSH-003 showed the possibility to produce 2-keto-gulonic acid at high yield (40 g/L) via one step fermentation (direct conversion of d-sorbitol to 2-keto-gulonic acid) by heterologous recombination of FAD-SDH and PQQ-GLDH genes derived from Ketogulonicigenium vulgare. In this study, the 2-keto-gulonic acid production yield was eightfold higher than that obtained using independent expression of the FAD-SDH and PQQ-GLDH dehydrogenases (Gao et al. 2014).
Bacterial cellulose
BC is a versatile biopolymer that can be used in a large variety of applications, including food (e.g. non-caloric bulker), pharmaceutical and biomedical products (e.g. cosmetics, skin substitute for burn wounds) and engineering (e.g. diaphragms for electro-acoustic transducers, paint additives, coatings and as reinforcement material) (Jonas and Farah 1998; Ku et al. 2011; Gullo et al. 2018).
The global BC market is estimated at 207.36 million USD in 2016 and is expected to reach 497.76 million USD by the end of 2022 (QYResearch Reports 2017). By the way, BC production at industrial scale is limited by the low yield and high production cost. The availability of suitable AAB strains and the optimization of both culture media and the production process seem to be key elements to overcome these limitations (Gullo et al. 2017; Ruka et al. 2012; Kuo et al. 2015b).
BC synthesis is highly variable within AAB, for example among K. xylinus strains isolated from Kombucha tea, the yield ranged from 0 to 12 g/L for the majority of strains. One strain (UMCC 2756) achieved a BC yield of over 23 g/L on a glucose-based medium (50 g/L) in 10 days of static cultivation (Gullo et al. 2017). On the other hand, Son and co-workers (2001) isolated a high BC-producing strain from apples (Acetobacter sp. A9), which achieved a BC yield of 15, 2 g/L using a medium at 40 g/L of glucose in agitated system.
Although the influence of carbon sources on BC production has been largely investigated, results seem discordant. Some authors reported the highest BC yield in glucose-only medium, whereas other obtained best results in media containing either mannitol or arabitol (Son et al. 2001; Oikawa et al. 1995a, b). Moreover, the addition of a small amount of ethanol to a glucose medium seems to stimulate BC production. This occurs since ethanol acts as an additional energy source, allowing the cells to use glucose mainly for BC synthesis (Krystynowicz et al. 2002; Gullo et al. 2017).
As described previously, AAB can use glucose to produce different compounds, such as gluconates and BC or to produce energy via glycolysis. To improve glucose utilization efficiency for BC production, Kuo et al. (2015a), generated the mutant strain K. xylinus GDH-KO in which the gene encoding for PQQ-GDH was knocked out through homologous recombination of a defective gdh gene. The mutant strain showed an increase in the BC production of about 40 and 230% compared to the wild-type strain in static and shaken culture, respectively. Furthermore, the gluconic acid formation in the mutant strain was negligible.
In order to reduce the costs of feedstock while contributing to environmental impact reduction, various low-cost alternative carbon sources have been evaluated. In particular, researches focused on relatively cheap agricultural products or waste containing glucose (Thompson and Hamilton 2001; Kuo et al. 2010). Although results are encouraging, many issues related to the yield and quality of the BC produced from these sources are still to be solved.
Fructans
The interest for fructans produced by bacteria arises from general properties of these compounds, like bio-compatibility, bio-degradability and biomedical properties such as antioxidant and anti-inflammatory. Fructans can be also used in cosmetic industry in the skin cream preparation and moisturizers. In the last years different studies focused fructans production by AAB, especially for food industry applications. The relevance of microbial fructans rather than plants fructans is due to their chemical-physical properties; in particular, the degree of polymerization is higher for microbial fructans compared to plant fructans (Srikanth et al. 2015).
Among fructans produced by AAB, LT-EPS are linear polymers that are useful in different fields. Molecular size of LT-EPS is the most relevant property that influences their functional characteristics such as rheological, technological and health properties (Benigar et al. 2014; Esawy et al. 2011). In food industries, LT-EPS have great potential as stabilizer, emulsifier, eco-friendly adhesive and bio-thickener, as well as anti-tumor and cholesterol-lowering agents (De Vuyst et al. 1998; Yoo et al. 2004). Moreover, they are considered prebiotic since their hydrolysis products, which are short-chain fructooligosaccharides (FOS), show the ability to preferentially stimulate the growth of intestinal bifidobacteria (Roberfroid et al. 1998).
So far, LT-EPS are seldom applied in the food industry due to the lack of commercially available preparations with defined structure. However, they are used in some commercial non-alcoholic beverages (e.g. in some ultra-high-fructose syrups) as sweetener or dietary fibre (Bello et al. 2001). Moreover, a promising application seems to be the use of LT-EPS to improve the dough rheology and taste of gluten-free (Ua-Arak et al. 2017; Jakob et al. 2012b). In particular, an increase of loaf volume and a reduction of bread staling rate were observed after the addition of LT-EPS produced by different AAB (Jakob et al. 2013). Comparative studies demonstrated that LT-EPS with higher molecular weight and branching at position 3 of the glucose monomer have a superior water-binding capacity and thus, affect more positively the bread characteristics (Ua-Arak et al. 2016; Rühmkorf et al. 2012).
Concerning nutritional and health-promoting aspects, it has been demonstrated, by in vivo studies that, LT-EPS exhibit antitumor even antiviral activities and anti-inflammatory properties by stimulating interferon response (Yoo et al. 2004; Esawy et al. 2011; Rairakhwada et al. 2007).
Last advance in the field of drug delivery systems is the development of polysaccharide-based nanometric carriers. LT-EPS are particularly suitable for this application since they have large number of reactive hydroxyl groups, high biocompatibility and self-degrading ability in human system (Srikanth et al. 2015). At this regard, thin-nanostructured films of pure and oxidized LT-EPS by matrix-assisted pulsed laser evaporation were developed. In vitro tests revealed high potential for cell proliferation especially for oxidized LT-EPS (Sima et al. 2011).
Conclusions and future directions
The use of AAB in bioprocesses spans from consolidated applications mainly linked to the oxidative fermentation pathways (acetic acid in vinegar production, gluconic acid, 2-keto-gulonic acid and dihydroxyacetone) to biopolymer production, such as BC and fructans, that although well studied at academic level, are still largely unexploited (Table 2).
The industry demand of cost-effective bio-based productions that comprises first high yield, no by-products formation and stability of strain performance during time in industrial conditions, are recognised as main factors limiting AAB exploitation. To fulfill the industry demand also established bioprocesses in which AAB are common applied, require further optimization, as in the case of acetic acid for vinegar production. Although there are many studies on strains improvement and processes optimization, the modern vinegar production is conducted via mixed AAB cultures. This system, on the one hand, allows to perform processes with low technological requirements and investments, on the other hand, it limits the full exploitation of the metabolic potential of selected strains. Considering both the requirements of the fermentation processes and the technological traits of selected strains, further growth of production yield is achievable by adopting rational strain selection approaches. Moreover, selection of strains could allow to differentiate marketable products, enhancing the portfolio of vinegars and related fermented beverages.
Gluconic acid and derivates, 2-keto-l-gulonic acid and dihydroxyacetone are industrially obtained by microbial fermentation using AAB, but obtaining higher production yield is still an open issue. Combining the use of selected wild and engineered strains with optimal process parameters is pivotal to overcome current limits.
Among emerging products from AAB, bacterial cellulose and fructans have received increasing attention because of their high potential in a wide array of applications, but actually the low production yield and high cost, limit the development of large-scale processes. However, the wide applied research in this area is providing the basic platform for optimization of industrial biopolymers processes using AAB.
The improvements of the use of AAB can be achieved by advance in molecular biology and genetic engineering researches that, at this time, provide the best approach in order to optimize and improve the production process and yield of the different bioproducts. Finally, since sustainability is a main industry issue, the optimization of processes from waste and low cost raw materials could further positively affect the industrial application of AAB.
References
Abdel-Fattah AM, Gamal-Eldeen AM, Helmy WA, Esawy MA (2012) Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr Polym 89:314–322
Adachi O, Yakushi T (2016) Membrane-bound dehydrogenases of acetic acid bacteria. In: Matsushita H, Toyama N, Tonouchi N, Okamoto-Kainuma A (eds) Acetic acid bacteria ecology and physiology. Springer, Japan, pp 273–297. https://doi.org/10.1007/978-4-431-55933-7_13
Adachi O, Tanasupawat S, Yoshihara N, Toyama H, Matsushita K (2003) 3-Dehydroquinate production by oxidative fermentation and further conversion of 3-dehydroquinate to the intermediates in the shikimate pathway. Biosci Biotechnol Biochem 67:2124–2131. https://doi.org/10.1271/bbb.67.2124
Ameyama M, Osada K, Shinagawa E, Matsushita K, Adachi O (1981) Purification and characterization of aldehyde dehydrogenase of Acetobacter aceti. Agric Biol Chem 45:1889–1890. https://doi.org/10.1271/bbb1961.45.1889
Ano Y, Shinagawa E, Adachi O, Toyama H, Yakushi T, Matsushita K (2011) Selective, high conversion of d-glucose to 5-keto-d-gluoconate by Gluconobacter suboxydans. Biosci Biotechnol Biochem 75:586–589. https://doi.org/10.1271/bbb.100701
Bartowsky EJ, Henschke PA (2008) Acetic acid bacteria spoilage of bottled red wine: a review. Int J Food Microbiol 125:60–70. https://doi.org/10.1016/j.ijfoodmicro.2007.10.016
Bello FD, Hertel C, Hammes W, Walter J (2001) In vitro study of prebiotic properties of levan-type exopolysaccharides from Lactobacilli and non-digestible carbohydrates using denaturing gradient gel electrophoresis. Syst Appl Microbiol 24:232–237. https://doi.org/10.1078/0723-2020-00033
Benigar E, Dogsa I, Stopar D, Jamnik A, Cigiæ IK, Tomšiè M (2014) Structure and dynamics of a polysaccharide matrix: aqueous solutions of bacterial levan. Langmuir 30:4172–4182. https://doi.org/10.1021/la500830j
Brandt JU, Jakob F, Jürgen B, Geissler AJ, Vogel RF (2016) Dissection of exopolysaccharide biosynthesis in Kozakia baliensis. Microb Cell Fact 15:170. https://doi.org/10.1186/s12934-016-0572-x
Cañete-Rodríguez AM, Santos-Dueñas IM, Jiménez JE, Ehrenreich HA, Liebl W, García-García I (2016) Gluconic acid: properties, production methods and applications-an excellent opportunity for agro-industrial by-products and waste bio-valorization. Process Biochem 51:1891–1903. https://doi.org/10.1016/j.procbio.2016.08.028
Chambert R, Treboul G, Dedonder R (1974) Kinetic studies of levansucrase of Bacillus subtilis. Eur J Biochem 41:285. https://doi.org/10.1111/j.1432-1033.1974.tb03269.x
Chozhavendhan S, Kumar RP, Elavazhagan S, Barathiraja B, Jayakumar M, Varjani SJ (2018) Utilization of crude glycerol from biodiesel industry for the production of value-added bioproducts. In: Singhania R, Agarwal R, Kumar R, Sukumaran R (eds) Waste to wealth. Energy, environment, and sustainability. Springer, Singapore, pp 65–82. https://doi.org/10.1007/978-981-10-7431-8_4
De Roos J, De Vuyst L (2018) Acetic acid bacteria in fermented foods and beverages. Curr Opin Microbiol 49:115–119. https://doi.org/10.1016/j.copbio.2017.08.007
De Vero L, Gullo M, Giudici P (2010) Acetic acid bacteria, biotechnological applications. Encycl Ind Biotechnol. https://doi.org/10.1002/9780470054581.eib003
De Vuyst L, Vanderveken F, Van de Ven S, Degeest B (1998) Production by and isolation of exopolysaccharides from Streptococcus thermophiles grown in milk medium and evidence for their growth-associated biosynthesis. J Appl Microbiol 84:1059–1068. https://doi.org/10.1046/j.1365-2672.1998.00445.x
Dikshit PK, Moholkar VS (2018) Batch and repeated-batch fermentation for 1,3-dihydroxyacetone production from waste glycerol using free, immobilized and resting Gluconobacter oxydans cells. Waste Biomass Valori. https://doi.org/10.1007/s12649-018-0307-9
Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and extraction. Carbohydr Polym 87:951–962. https://doi.org/10.1016/j.carbpol.2011.08.083
Esawy MA, Ahmed EF, Helmy WA, Mansour NM, El-Senousy WM, El-Safty MM (2011) Production of levansucrase from novel honey Bacillus subtilis isolates capable of producing antiviral levans. Carbohydr Polym 86:823–830. https://doi.org/10.1016/j.carbpol.2011.05.035
Gao L, Hu Y, Liu J, Du G, Zhou J, Chen J (2014) Stepwise metabolic engineering of Gluconobacter oxydans WSH-003 for the direct production of 2-keto-l-gulonic acid from d-sorbitol. Metab Eng 24:30–37. https://doi.org/10.1016/j.ymben.2014.04.003
García-García I, Cañete-Rodríguez AM, Santos-Dueñas IM, Jiménez Hornero JE, Ehrenreich A, Liebl W, García-Martínez T, Mauricio JC (2017) Biotechnologically relevant features of gluconic acid production by acetic acid bacteria. Acetic Acid Bact 6(6458):7–12. https://doi.org/10.4081/aab.2017.6458
Gätgens C, Degner U, Bringer-Meyer S, Herrmann U (2007) Biotransformation of glycerol to dihydroxyacetone by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 76:553–559. https://doi.org/10.1007/s00253-007-1003-z
Giudici P, Gullo M, Solieri L (2009) Traditional balsamic vinegar. In: Solieri L, Giudici P (eds) Vinegars of the world. Springer, Milano, pp 157–177. https://doi.org/10.1007/978-88-470-0866-3_10
Giudici P, De Vero L, Gullo M, Solieri L, Lemmetti F (2016) Fermentation strategy to produce high gluconate vinegar. Acetic Acid Bact 5(6067):1–6. https://doi.org/10.4081/aab.2016.6067
Gullo M, Giudici P (2008) Acetic acid bacteria in traditional balsamic vinegar: phenotypic traits relevant for starter cultures selection. Int J Food Microbiol 125:46–53. https://doi.org/10.1016/j.ijfoodmicro.2007.11.076
Gullo M, De Vero L, Giudici P (2009) Succession of selected strains of Acetobacter pasteurianus and other acetic acid bacteria in traditional balsamic vinegar. Appl Environ Microbiol 75:2585–2589. https://doi.org/10.1128/AEM.02249-08
Gullo M, Verzelloni E, Canonico M (2014) Aerobic submerged fermentation by acetic acid bacteria for vinegar production: process and biotechnological aspects. Process Biochem 49:1571–1579. https://doi.org/10.1016/j.procbio.2014.07.003
Gullo M, Zanichelli G, Verzelloni E, Lemmetti F, Giudici P (2016) Feasible acetic acid fermentations of alcoholic and sugary substrates in combined operation mode. Process Biochem 51:1129–1139. https://doi.org/10.1016/j.procbio.2016.05.018
Gullo M, Sola A, Zanichelli G, Montorsi M, Messori M, Giudici P (2017) Increased production of bacterial cellulose as starting point for scaled-up applications. Appl Microbiol Biotechnol 101:8115–8127. https://doi.org/10.1007/s00253-017-8539-3
Gullo M, La China S, Falcone PM, Giudici P (2018) Biotechnological production of cellulose by acetic acid bacteria: current state and perspectives. Appl Microbial Biotechnol. https://doi.org/10.1007/s00253-018-9164-5
Han YW, Clarke MA (1990) Production and characterization of microbial levan. J Agric Food Chem 38:393–396. https://doi.org/10.1021/jf00092a011
Hekmat D, Bauer R, Fricke J (2003) Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess Biosyst Eng 26:109–116. https://doi.org/10.1007/s00449-003-0338-9
Herrmann U, Merfort M, Jeude M, Bringer-Meyer S, Sahm H (2004) Biotransformation of glucose to 5-keto-d-gluconic acid by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 64:86–90. https://doi.org/10.1007/s00253-003-1455-8
Hidalgo C, Vegas C, Mateo E, Tesfaye W, Cerezo AB, Callejón RM, Poblet M, Guillamón JM, Mas A, Torija MJ (2010) Effect of barrel design and the inoculation of Acetobacter pasteurianus in wine vinegar production. Int J Food Microbiol 141:56–62. https://doi.org/10.1016/j.ijfoodmicro.2010.04.018
Hoshino T, Sugisawa T, Tazoe M, Shinjoh M, Fujiwara A (1990) Metabolic pathway for 2-keto-l-gulonic acid formation in Gluconobacter melanogenus IFO 3293. Agric Biol Chem 54:1211–1218. https://doi.org/10.1080/00021369.1990.10870126
Jakob F, Meissner D, Vogel RF (2012a) Comparison of novel GH 68 levansucrases of levan-overproducing Gluconobacter species. Acetic Acid Bact 1:e2. https://doi.org/10.4081/aab.2012.e2
Jakob F, Steger S, Vogel RF (2012b) Influence of novel fructans produced by selected acetic acid bacteria on the volume and texture of wheat breads. Eur Food Res Technol 234(3):493–499. https://doi.org/10.1007/s00217-011-1658-7
Jakob F, Phaff A, Novoa-Carballal R, Rübsam H, Becker T, Vogel RF (2013) Structural analysis of fructans produced by acetic acid bacteria reveals a relation to hydrocolloidal function. Carbohydr Polym 92:1234–1242. https://doi.org/10.1016/j.carbpol.2012.10.054
Jiménez-Hornero JE, Santos-Dueñas IM, García-García I (2009) Optimization of biotechnological processes. The acetic acid fermentation. Part I: the proposed model. Biochem Eng J 45:1–6. https://doi.org/10.1016/j.bej.2009.01.009
Jonas R, Farah LF (1998) Production and application of microbial cellulose. Polym Degrad Stabil 59:101–106. https://doi.org/10.1016/S0141-3910(97)00197-3
Krystynowicz A, Czaja W, Wiktorowska-Jezierska A, Gonçalves-Miśkiewicz M, Turkiewicz M, Bielecki S (2002) Factors affecting the yield and properties of bacterial cellulose. J Ind Microbiol Biotechnol 29:189–195. https://doi.org/10.1038/sj.jim.7000303
Ku H, Wang H, Pattarachaiyakoop N, Trada M (2011) A review of tensile properties of natural fiber reinforced polymer composites. Compos B 42:856–873. https://doi.org/10.1016/j.compositesb.2011.01.010
Kuo CH, Lin PJ, Lee CK (2010) Enzymatic saccharification of dissolution pretreated waste cellulosic fabrics for bacterial cellulose production by Gluconacetobacter xylinus. J Chem Technol Biotechnol 85:1346–1352. https://doi.org/10.1002/jctb.2439
Kuo CH, Chen JH, Liou BK, Lee CK (2015a) Utilization of acetate buffer to improve bacterial cellulose production by Gluconacetobacter xylinus. Food Hydrocoll 53:98–103. https://doi.org/10.1016/j.foodhyd.2014.12.034
Kuo CH, Teng HY, Lee CK (2015b) Knock-out of glucose dehydrogenase gene in Gluconacetobacter xylinus for bacterial cellulose production enhancement. Biotechnol Bioprocess E 20:18–25
Li MH, Wu J, Liu X, Lin JP, Wei DZ, Chen H (2010) Enhanced production of dihydroxyacetone from glycerol by overexpression of glycerol dehydrogenase in an alcohol dehydrogenase-deficient mutant of Gluconobacter oxydans. Bioresour Technol 101:8294–8299. https://doi.org/10.1016/j.biortech.2010.05.065
Li K, Mao X, Liu L, Lin J, Sun M, Wei D, Yang S (2016) Overexpression of membrane-bound gluconate-2-dehydrogenase to enhance the production of 2-keto-d-gluconic acid by Gluconobacter oxydans. Microb Cell Fact 15:121. https://doi.org/10.1186/s12934-016-0521-8
Lu L, Wei L, Zhu K, Wei D, Hua Q (2012) Combining metabolic engineering and adaptive evolution to enhance the production of dihydroxyacetone from glycerol by Gluconobacter oxydans in a low-cost way. Bioresour Technol 17:317–324. https://doi.org/10.1016/j.biortech.2012.03.013
Lux S, Siebenhofer M (2013) Synthesis of lactic acid from dihydroxyacetone: use of alkaline-earth metal hydroxides. Catal Sci Technol 3:1380–1385. https://doi.org/10.1039/C3CY20859A
Macauley S, McNeil B, Harvey LM (2001) The genus Gluconobacter and its applications in biotechnology. Crit Rev Biotechnol 21:1–25. https://doi.org/10.1080/20013891081665
Mamlouk D, Gullo M (2013) Acetic acid bacteria: physiology and carbon sources oxidation. Indian J Microbiol 53:377–384. https://doi.org/10.1007/s12088-013-0414-z
Mas A, Torija MJ, García-Parrilla MC, Troncoso AM (2014) Acetic acid bacteria and the production and quality of wine vinegar. Sci World J. https://doi.org/10.1155/2014/394671
Matsushita K, Toyama H, Adachi O (1994) Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microb Physiol 36:247–301. https://doi.org/10.1016/S0065-2911(08)60181-2
Matsutani M, Nishikura M, Saichana N, Hatano T, Masud-Tippayasak U, Theergool G, Yakushi T, Matsushita K (2013) Adaptive mutation of Acetobacter pasteurianus SKU1108 enhances acetic acid fermentation ability at high temperature. J Biotechnol 165:109–119. https://doi.org/10.1016/j.jbiotec.2013.03.006
Mendez C, Salas JA (2001) The role of ABC transporters in antibiotic-producing organisms: drug secretion and resistance mechanisms. Res Microbiol 152:341–350. https://doi.org/10.1016/S0923-2508(01)01205-0
Méndez-Lorenzo L, Porras-Domínguez JR, Raga-Carbajal E, Olvera C, Rodríguez-Alegría ME, Carrillo-Nava E, Costas M, López Munguía A (2015) Intrinsic levanase activity of Bacillus subtilis 168 Levansucrase (SacB). ed. PLoS ONE 10:e0143394. https://doi.org/10.1371/journal.pone.0143394
Merfort M, Herrmann U, Bringer-Meyer S, Sahm H (2006) High-yield 5-keto-d-gluconic acid formation is mediated by soluble and membrane-bound gluconate-5-dehydrogenases of Gluconobacter oxydans. Appl Microbiol Biotechnol 73:443–451. https://doi.org/10.1007/s00253-006-0467-6
Muongman P, Opasanon S, Suwanchot S, Thangthed O (2011) Efficiency of microbial cellulose dressing in partial-thickness burn wounds. J Am Coll Cert Wound Special 3:16–19. https://doi.org/10.1016/j.jcws.2011.04.001
Murali N, Fernandez S, Ahring BK (2017) Fermentation of wet-exploded corn stover for the production of volatile fatty acids. Bioresour Technol 227:197–204. https://doi.org/10.1016/j.biortech.2016.12.012
Ndoye B, Lebecque S, Dubois-Dauphin R, Tounkara L, Guiro AT, Kere C, Diawara B, Thonart P (2006) Thermoresistant properties of acetic acids bacteria isolated from tropical products of Sub-Saharan Africa and destined to industrial vinegar. Enzyme Microb Technol 39:916–923. https://doi.org/10.1016/j.enzmictec.2006.01.020
Oikawa T, Morino T, Ameyama M (1995a) Production of cellulose from n-arabitol by Acetobacter xylinum KU-l. Biosci Biotechnol Biochem 59:1564–1565. https://doi.org/10.1271/bbb.59.1564
Oikawa T, Ohtori T, Ameyama M (1995b) Production of cellulose from n-mannitol by Acetobacter xylinum KU-l. Biosci Biotechnol Biochem 59:331–332. https://doi.org/10.1271/bbb.59.331
Okamoto-Kainuma A, Ishikawa M, Nakamura H, Fukazawa S, Tanaka N, Yamagami K, Koizumi Y (2011) Characterization of rpoH in Acetobacter pasteurianus NBR3283. J Biosci Bioeng 111:429–432. https://doi.org/10.1016/j.jbiosc.2010.12.016
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Pina CD (2007) From glycerol to value-added products. Angew Chem 46:4434–4440. https://doi.org/10.1002/anie.200604694
Pappenberger G, Hohmann HP (2014) Industrial production of l-ascorbic acid (vitamin C) and d-isoascorbic acid. Adv Biochem Eng Biotechnol 143:143–188. https://doi.org/10.1007/10_2013_243
Qi Z, Wang W, Yang H, Xia X, Yu X (2013) Mutation of Acetobacter pasteurianus by UV irradiation under acidic stress for high-acidity vinegar fermentation. Int J Food Sci Technol 49:468–476. https://doi.org/10.1111/ijfs.12324
QYResearch Reports (2017). https://www.qyresearchreports.com/. Accessed 5 April 2018
Rairakhwada D, Pal AK, Bhathena ZP, Sahu NP, Jha A, Mukherjee SC (2007) Dietary microbial levan enhances cellular non-specific immunity and survival of common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol 22:477–486. https://doi.org/10.1016/j.fsi.2006.06.005
Roberfroid MB, Van Loo JA, Gibson GR (1998) The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 128:11–19. https://doi.org/10.1093/jn/128.1.11
Roehr M, Kubicek CP, Kominek J (1996) Gluconic acid. In: Rehm HJ, Reed G (eds) Biotechnology, product of primary metabolism. Verlag Chemie, Weinheim, pp 347–362. https://doi.org/10.1002/9783527620999.ch10f
Rogers P, Chen JS, Zidwick MJ (2006) Organic acid and solvent production Part I: Acetic, lactic, gluconic, succinic and polyhydroxyalkanoic acids. In: Dworkin M (ed) Prokaryotes. Springer, New York, pp 511–755. https://doi.org/10.1007/978-3-642-31331-8_23
Ross P, Mayer R, Benziman M (1991) Cellulose biosynthesis and function in bacteria. Microbiol Rev 55:35–58
Rühmkorf C, Jungkunz S, Wagner M, Vogel RF (2012) Optimization of homoexopolysaccharide formation by lactobacilli in gluten-free sourdoughs. Food Microbiol 32:286–294. https://doi.org/10.1016/j.fm.2012.07.002
Ruka D, Simon GP, Dean KM (2012) Altering the growth conditions of Gluconacetobacter xylinus to maximize the yield of bacterial cellulose. Carbohydr Polym 89:613–622. https://doi.org/10.1016/j.carbpol.2012.03.059
Saichana N, Matsushita K, Adachi O, Frebort I, Frebortova J (2015) Acetic acid bacteria: a group of bacteria with versatile biotechnological applications. Biotechnol Adv 33:1260–1271. https://doi.org/10.1016/j.biotechadv.2014.12.001
Sainz F, Navarro D, Mateo E, Torija MJ, Mas A (2016) Comparison of d-gluconic acid production in selected strains of acetic acid bacteria. Int J Food Microbiol 222:40–47. https://doi.org/10.1016/j.ijfoodmicro.2016.01.015
Saito Y, Ishii Y, Hayashi H, Imao Y et al (1997) Cloning of genes coding for l-sorbose and l-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-l-gulonate, a precursor of l-ascorbic acid, in a recombinant G. oxydans strain. Appl Environ Microbiol 63:454–460
Sengun Y, Karabiyikli S (2011) Importance of acetic acid bacteria in food industry. Food Control 22:647–656. https://doi.org/10.1016/j.foodcont.2010.11.008
Shi L, Li K, Zhang H, Liu X, Lin J, Wei D (2014) Identification of a novel promoter gHp0169 for gene expression in Gluconobacter oxydans. J Biotechnol 175:69–74. https://doi.org/10.1016/j.jbiotec.2014.01.035
Shinagawa E, Matsushita K, Adachi O, Ameyama M (1983) Selective production of 5-keto-d-gluconate by Gluconobacter strains. J Ferment Technol 61:359–363
Shinjoh M, Toyama H (2016) Industrial application of acetic acid bacteria (Vitamin C and others). In: Matsushita K, Toyama H, Tonouchi N, Okamoto-Kainuma A (eds) Acetic acid bacteria ecology and physiology. Springer, Japan, pp 321–338. https://doi.org/10.1007/978-4-431-55933-7_15
Sima F, Mutlu EC, Eroglu MS, Sima LE, Serban N, Ristoscu C, Petrescu SM, Oner ET, Mihailescu IN (2011) Levan nanostructured thin films by MAPLE assembling. Biomacromol 12:2251–2256. https://doi.org/10.1021/bm200340b
Singh OV, Kumar R (2007) Biotechnological production of gluconic acid: future implications. Appl Microbiol Biotechnol 75:713–722. https://doi.org/10.1007/s00253-007-0851-x
Sokollek SJ, Hammes WP (1997) Description of a starter culture preparation for vinegar fermentation. Syst Appl Microbiol 20:481–491. https://doi.org/10.1016/S0723-2020(97)80017-3
Son HJ, Heo MS, Kim YG, Lee SJ (2001) Optimization of fermentation conditions for the production of bacterial cellulose by a newly isolated Acetobacter sp. A9 in shaking cultures. Biotechnol Appl Biochem 33:1–5. https://doi.org/10.1042/BA20000065
Sonoyama T, Tani H, Matsuda K, Kageyama B, Tanimoto M, Kobayashi K, Yagi S, Kyotani H, Mitsushima K (1982) Production of 2-keto-l-gulonic acid from d-glucose by two-stage fermentation. Appl Environ Microbiol 43:1064–1069
Srikanth R, Siddartha G, Sundhar Reddy CH, Harish BS, Janaki Ramaiah M, Uppuluri KB (2015) Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM2526 and its statistical optimization. Carbohydr Polym 123:8–16. https://doi.org/10.1016/j.carbpol.2014.12.079
Stottmeister U, Aurich A, Wilde H, Andersch J, Schemidt S, Sicker D (2005) White biotechnology for green chemistry: fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. J Ind Microbiol Biotechnol 32:651. https://doi.org/10.1007/s10295-005-0254-x
Sugisawa T, Hoshino T, Nomura S, Fujiwara A (1991) Isolation and characterization of membrane-bound l-sorbose dehydrogenase from Gluconobacter melanogenus UV10. Agric Biol Chem 55:363–370. https://doi.org/10.1080/00021369.1991.10870609
Thompson DN, Hamilton MA (2001) Production of bacterial cellulose from alternate feedstocks. Appl Biochem Biotechnol 91–93:503–513. https://doi.org/10.1385/ABAB:91-93:1-9:503
Toyama H, Soemphol W, Moonmangmee D, Adachi O, Matsushita K (2005) Molecular properties of membrane-bound FAD-containing D-sorbitol dehydrogenase from thermotolerant Gluconobacter frateurii isolated from Thailand. Biosci Biotechnol Biochem 69:1120–1129. https://doi.org/10.1271/bbb.69.1120
Ua-Arak T, Jakob F, Vogel RF (2016) Characterization of growth and exopolysaccharide production of selected acetic acid bacteria in buckwheat sourdoughs. Int J Food Microbiol 239:103–112. https://doi.org/10.1016/j.ijfoodmicro.2016.04.009
Ua-Arak T, Jakob F, Vogel RF (2017) Modulation of size distributions and functional properties in gluten-free baking of Gluconobacter albidus TMW 2.1191 levan in fermentations with controlled pH. Front Microbiol 8:807. https://doi.org/10.3389/fmicb.2017.00807
Vidra A, Németh Á (2018) Bio-produced acetic acid: a review. Period Polytech Chem 62(3):245–256. https://doi.org/10.3311/PPch.11004
Wei SH, Song QX, Wei DZ (2007) Repeated use of immobilized Gluconobacter oxydans cells for conversion of glycerol to dihydroxyacetone. Prep Biochem Biotechnol 37:67–76. https://doi.org/10.1080/10826060601040954
Wu JJ, Gullo M, Chen FS, Giudici P (2010) Diversity of Acetobacter pasteurianus strains isolated from solid-state fermentation of cereal vinegars. Curr Microbiol 60:280–286. https://doi.org/10.1007/s00284-009-9538-0
Xu S, Wang W, Du G, Zhou J, Chen J (2014) Enhanced production of l-sorbose from d-sorbitol by improving the mRNA abundance of sorbitol dehydrogenase in Gluconobacter oxydans WSH-003. Microb Cell Fact 13:146. https://doi.org/10.1186/s12934-014-0146-8
Yoo S, Yoon E, Cha E, Lee H (2004) Antitumor activity of levan polysaccharides from selected microorganisms. Int J Biol Macromol 34:37–41. https://doi.org/10.1016/j.ijbiomac.2004.01.002
Zheng Y, Wang J, Bai X, Chang Y, Mou J, Song J, Min W (2018) Improving the acetic acid tolerance and fermentation of Acetobacter pasteurianus by nucleotide excision repair protein UvrA. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-018-9066-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
La China, S., Zanichelli, G., De Vero, L. et al. Oxidative fermentations and exopolysaccharides production by acetic acid bacteria: a mini review. Biotechnol Lett 40, 1289–1302 (2018). https://doi.org/10.1007/s10529-018-2591-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-018-2591-7