Abstract
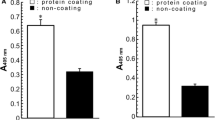
Bovine lactoferrin promotes bifidobacterial growth. Its binding to bifidobacteria is thought to be responsible for such action. After separating the bovine lactoferrin half molecule and extraction of surface proteins from bifidobacteria, binding profiles were observed by immunoblotting. No binding appeared when lactoferrin C-lobe was reacted with the cell surface proteins on a polyvinylidene difluoride membrane. Conversely, a 50-kDa band appeared when the surface proteins were reacted with either intact or nicked bovine lactoferrin. This result strongly suggests that the binding region could be lactoferrin N-lobe. Interestingly, despite the absence of binding, C-lobe enhanced bifidobacterial growth.



Similar content being viewed by others
References
Almeida RA, Luther DA, Park H-M, Oliver SP (2006) Identification, isolation, and partial characterization of a novel Streptococcus uberis adhesion molecule (SUAM). Vet Microbiol 115:183–191
Baker EN, Baker HM, Kidd RD (2002) Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem Cell Biol 80:27–34
Bellamy W, Takase M, Yamauchi K, Wakabayashi H, Kawase K, Tomita M (1992) Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta 1121:130–136
Bezkorovainy A, Kot E (1998) Interaction of bifidobacteria with ferric iron. Int Dairy J 8:507–512
Bonnah RA, Schryvers AB (1998) Preparation and characterization of Neisseria meningitidis mutants deficient in the production of the human lactoferrin binding proteins LbpA and LbpB. J Bacteriol 180:3080–3090
Gonzáles-Chávez SA, Arévalo-Galleyegos S, Rascón-Cruz Q (2008) Lactoferrin: Structure, function and applications. Int J Antimicrob Agents. doi:10.1016/j.ijantimicag.2008.07.020 (in press)
Kim W-S, Ohashi M, Tanaka T, Kumura H, Kim G-Y, Kwon I-K, Shimazaki K (2004) Growth-promoting effects of lactoferrin on L. acidophilus and Bifidobacterium spp. BioMetals 17:279–283
Liepke C, Adermann K, Raida M, Magert HJ, Forssmann WG, Zucht HD (2002) Human milk provides peptides highly stimulating the growth of bifidobacteria. Eur J Biochem 269:712–718
Miller-Catchpole R, Kot E, Haloftis G, Furmanov S, Bezkorovainy A (1997) Lactoferrin can supply iron for the growth of Bifidobacterium breve. Nutr Res 17:205–213
Mitsuoka T (1990) Bifidobacteria and their role in human health. J Indust Microbiol 6:263–268
Petschow BW, Talbott RD, Batema RP (1999) Ability of lactoferrin to promote the growth of Bifidobacterium spp. in vitro is independent of receptor binding capacity and iron saturation level. J Med Microbiol 48:541–549
Rochard E, Legrand D, Majurier J, Montreuil J, Spik G (1989) The N-terminal domain I of human lactotransferrin binds specifically to phytohemagglutinin-stimulated peripheral blood human lymphocyte receptors. FEBS Lett 255:201–204
Shimazaki K (2000) Lactoferrin: A marvelous protein in milk? Anim Sci J 71:329–347
Shimazaki K, Tanaka T, Kon H, Oota K, Kawaguchi A, Maki Y, Sato T (1993) Separation and Characterization of the C-terminal half molecule of bovine lactoferrin. J Dairy Sci 76:946–955
Sitaram MP, McAbee DD (1997) Isolated rat hepatocytes differentially bind and internalize bovine lactoferrin N- and C-lobes. Biochem J 323:815–822
Takayama Y, Mizumachi K, Takezawa T (2002) The bovine lactoferrin region responsible for promoting the collagen gel contractile activity of human fibroblasts. Biochem Biophys Res Commun 299:813–817
van Berkel PH, Geerts ME, van Veen HA, Mericskay M, Boer HA, Nuijens JH (1997) N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J 328:145–151
van der Kraan MI, Groenink J, Nazmi K, Veerman EC, Bolscher JG, Nieuw Amerongen AV (2004) Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides 25:177–183
Ward PP, Zhou X, Conneely OM (1996) Cooperative interactions between the amino- and carboxyl-terminal lobes contribute to the unique iron-binding stability of lactoferrin. J Biol Chem 271:12790–12794
Wong H, Schryvers AB (2003) Bacterial lactoferrin binding protein A binds to both domains of the human lactoferrin C-lobe. Microbiology 149:1729–1737
Acknowledgement
The authors express their sincere appreciation to Miss Tomomi Yokoyama for separating the bovine lactoferrin half molecule which greatly facilitated this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M., Kim, WS., Kumura, H. et al. Bovine lactoferrin region responsible for binding to bifidobacterial cell surface proteins. Biotechnol Lett 31, 863–868 (2009). https://doi.org/10.1007/s10529-009-9936-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-9936-1