Abstract
Astaxanthin (AX) is one of the commonly used feed supplements to enhance the growth performance and provide antioxidant and immune functions of several aquatic animals. In the current study, juveniles of white-leg shrimp (Litopenaeus vannamei) with mean initial weight of 0.340 ± 0.041 g were fed with diets supplemented with 0 (control), 25, 50, 100, and 200 mg/kg feed for 8 weeks. At the end of the feeding trial, shrimps were exposed to Vibrio harveyi, and their mortality rates were observed for additional 10 days. The growth indices in the AX-fed groups were significantly (P < 0.05) higher than what were observed in shrimps in the control group. Dietary AX stimulated the final weight, weight gain, and specific growth rate and optimum growth levels were achieved at 100–200 mg AX/kg feed. Furthermore, the AX-enriched diets significantly enhanced feed intake more than the control diet, and the amount of AX had no effects on feed conversion ratios. In comparison to the control group, the AX-fed animals had significantly (P < 0.05) higher villi length, villi width, and absorption area and their optimum values were observed at 100–200 mg AX/kg feed treatments. Moreover, the intestinal morphometry especially villi and its crypt, both internal and external tunica muscularis, and submucosal tissues did not show any inflammatory and/or degenerative changes in AX-fed shrimp. Furthermore, the dietary AX at escalating levels linearly and quadratically enhanced (P < 0.05) the activities of serum superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and increased levels of total antioxidant capacity and reduced glutathione. In addition, malondialdehyde levels decreased significantly in AX-fed animals, and the highest levels were observed in the control group (without AX). The expression levels of cMn-SOD, CAT, and GPx genes were significantly upregulated in the hepatopancreas of L. vannamei fed with AX-enriched diets (especially in the 200-mg/kg feed treatment) as compared with the control diet. The immunity indices of the AX treatments (hematocyte count, total protein, lysozyme, phagocytic activity, and phenoloxidase) of L. vannamei were linearly (P < 0.05) and quadratically (P < 0.05) increased. This study revealed the antibacterial activity of AX against V. harveyi abundance. After the bacterial challenge, feeding L. vannamei with dietary AX significantly increased (P < 0.05) the relative percentage of survival, especially in the treatment of 200-mg/kg diet (82.7%). The findings of the current study demonstrate that dietary AX (100–200 mg/kg feed) was effective in enhancing the growth, antioxidant status, immune response, and increasing the resistance of L. vannamei against V. harveyi infection.
Similar content being viewed by others
Introduction
Shrimps are widely distributed throughout the world and its aquaculture constitutes a substantial proportion of the aquaculture economy in numerous countries. One of the most profitable shrimp species, white-leg shrimp (Litopenaeus vannamei), is widely distributed and has become a highly valuable commodity (FAO 2020). However, intensive farming of this shrimp species often involves high-stress environments and is, therefore, impeded by occurrences such as bacterial infections, for example, Vibrio harveyi. Luminous disease, caused primarily by V. harveyi, constitutes a severe threat to penaeid shrimp farming, because it frequently leads to quick and substantial shrimp mortality (Teo et al. 2000; Austin and Zhang 2006). To prevent economic losses, protecting shrimp against bacterial infections is crucial. Traditionally, antibiotics/chemotherapeutics are commonly used to control vibriosis (Laganà et al. 2011; Yano et al. 2014). However, its usage in aquaculture has been criticized, because of emergence of antibiotic-resistant bacterial strains, residual effects, inducement of adverse health in consumers, and environment (Maron et al. 2013; Adel et al. 2017; Santos and Ramos 2018). Therefore, finding effective, safe, and eco-friendly alternatives to antibiotics has become increasingly important. Among various possible treatments, natural disease-controlling supplements in aquafeeds are the most promising alternatives to prophylactic antibiotics. Various natural substances stimulate growth, increase antioxidants, and improve the immunity of aquatic animals. In addition, because of their bioactive compounds, they could control bacterial infections in many aquatic organisms (Van Hai 2015; Dawood et al. 2018, 2020; Alagawany et al. 2020) including vibrosis (Yilmaz et al. 2022).
Various plants, microalgae, and microbes are capable of synthesizing a bright-red carotenoid called astaxanthin (AX) (Ho et al. 2018; Jin et al. 2018) which has been approved by the United States Food and Drug Administration as a food colorant in animal and fish feeds (Pashkow et al. 2008). In aquaculture, it has valuable applications as a feed supplement because of its biological functions including skin and flesh pigmentation, antioxidant activity, immune enhancement, stress alleviation, growth stimulation, and mortality reduction (Li et al. 2020; Wang et al 2020; Lim et al. 2021). The development of shrimps’ aquaculture continues to increase the demand for dietary AX (Chew 1995). Because AX aids in enhancing the growth indices and supporting antioxidant capacity and innate immunity, its use in aquafeeds could promote sustainable development of shrimp aquaculture. Therefore, the present study investigated the impacts of dietary AX on the growth indices, antioxidant status, immune response, and disease resistance of white-leg shrimp (L. vannamei) to V. harveyi.
Materials and methods
Antibacterial activity of AX against V. harveyi.
AX (C40H52O4; MW = 483.51) was purchased as Carophyll ® pink 10% DSM (Vita lucantin pink 10%, Bangkok, Thailand). The disk diffusion method was used to test the antagonistic activity of AX against V. harveyi. A 24-h inoculum suspension of V. harveyi was uniformly swabbed on solidified thiosulfate-citrate-bile salt-sucrose agar media (TCBS) (Merck, Darmstadt, Germany). The bacteria inoculum was allowed to dry for 5 min. Filter-paper disks (6 mm) were made using a hole punch and placed on an agar. Aliquots of 10 mL from AX were added to sterilized petri dishes. Then, filter-paper disks were soaked in various concentrations of AX for 15 min, transferred to the seeded medium, and allowed to remain on the bench for sufficient diffusion. All samples were prepared in triplicates to obtain means. After 24-h incubation at 28 °C, the resulted inhibition zones were measured in millimeters.
Diet formulation and preparation
The experimental diets (40% crude protein) were prepared, and AX was added at levels of 0.0 (control), 25, 50, 100, and 200 mg/kg to the diet. For each diet, the respective proportion of AX was mixed with soybean oil and subsequently mixed with the remaining feed ingredients; then, water was added (100 mL per kg diet) and well mixed for 30 min. All these steps involved thorough mixing of the ingredients. Finally, the diets were pelletized (approximately 0.5-mm diameter), dried in the dark, and stored at − 4 °C. This is to prevent degradation of AX in the feed. The formulation and proximate composition of the control diet is provided in Table 1.
Shrimp rearing
White-leg shrimps (mean initial weight = 0.340 ± 0.041 g) were acquired from a shrimp hatchery in the Ghalioun Project, Egypt, and then acclimated to the subsequent culture environment in oxygenated seawater (32‰ salinity) for 2 weeks. During this period, the shrimp were fed with the control diet (40% CP). Healthy shrimps were randomly divided into 15 1-m3 cylindrical fiberglass tanks where each tank contained 300 individuals and each treatment was replicated three times. Shrimps were given the experimental diets to apparent satiety at 8:00, 12:30, 16:00, and 20:00 h for 8 weeks. Every 2 days, feces were removed together with 50% of the water using a siphon tube, and the removed water was replaced with new seawater.
The water quality was monitored daily. Water pH (7.8–8.2), salinity (22–23‰), temperature (28–31 °C), ammonia–nitrogen (0.06–0.08 mg/L), and dissolved oxygen (6.5–6.8 mg/L) were recorded weekly throughout the experimental period in accordance with the APHA standard methods (APHA 1998).
After the feeding trial, all the shrimps in each tank were harvested, counted, and weighed. Parameters of growth and feed utilization were calculated according to the following formulae:
Weight gain (%) = 100 [final weight (g) − initial weight (g)] / initial weight (g);
Specific growth rate (SGR; %/day) = 100 × (Ln final weight − Ln initial weight) / experimental days;
Feed conversion ratio (FCR) = feed intake (g) / weight gain (g);
Animal survival (%) = 100 × [animal number at the final − animal number at the start].
Hemolymph and tissue sampling
After 8 weeks, 10 animals from each tank (30 animals per treatment) were removed and fasted for 24 h. Hemolymph was collected from the ventral sinus, allowed to coagulate, kept at 4 °C for 24 h, and then repeatedly centrifuged at 2000 × g to obtain the serum, which was aliquoted and stored at − 20 °C until further use. Another part was withdrawn using a syringe containing 750 μL of precooled (4 °C) anticoagulant (0.114 M trisodium citrate, 450 mM NaCl, 10 mM KCl, and 10 mM HEPES at pH 7.4). After hemolymph sampling, animals were dissected, and hepatopancreas tissues were removed and stored in liquid nitrogen (− 80 °C) for future analysis and determination of the genes expression.
Intestinal morphometric analysis
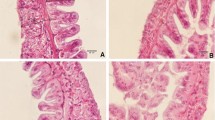
The intestinal tract of 10 animals/tank from the different groups were collected at the end of the experiment (8 weeks). The samples were first fixed in 4% buffered formalin for 24 h, then the tissues were dehydrated by immersing them in 70%, 85%, and 98% alcohol, before finally embedding them in paraffin. Histological Sections 4–5 µm thick were sliced using a microtome (Leica, Germany), and sections were stained with hematoxylin and eosin to observe the general morphology of the samples. Images were photographed using an Olympus BX50 microscope (Japan) (Roberts and Smail 2001).
Antioxidant activity and lipid peroxidation
Commercial assay kits from Biodiagnostic Co., Cairo, Egypt were used to measure the levels of enzymes activity and lipid peroxidation. The SOD activity was measured following the method of McCord and Fridovich (1969). Following the addition of 10 μL of serum to the kits reagent, catalase (CAT) activity was measured from the decrease in H2O2 concentration (Aebi 1984). Glutathione peroxidase (GPx) activity was measured following the method of Flohé and Günzler (1984). The reduced GSH was measured according to the method of Browne and Armstrong (1998). The level of malondialdehyde (MDA) during oxidizing of fatty acids was used to determine the lipid peroxidation levels according to Ohkawa et al. (1979). The total antioxidant capacity (TAC) of serum was determined using the ferric-reducing antioxidant power (FRAP) method (Benzie and Strain 1996).
Antioxidant gene expression
The expression levels of cytosolic manganese superoxide dismutase (cMn-SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured in hepatopancreatic tissue of L. vannamei fed with different levels of AX over the 8-week feeding trial. The hepatopancreas samples were centrifuged at 3000 × g for 10 min at 4 °C and the supernatant immediately stored at − 80 °C for later assay of gene expression. Total RNA was extracted from samples using TRIzol reagents (Invitrogen, UK) according to the manufacturer’s instructions. The RNA was reverse transcribed by RT-PCR using a SYBR Green method in an iQ5 iCycler thermal cycler (Bio-Rad, Hercules, CA, USA). The reactions were performed in a 96-well plate, with each reaction containing 1 µL of diluted (1/20) cDNA, 5 µL of concentrated iQ TM SYBR Green Supermix (Bio-Rad) as a fluorescent intercalating agent, 0.3 µM forward primer, and 0.3 µM of the reverse primer. The sequences of each primer used in the present study were designed based on the published L. vannamei cDNA sequence (Table 2) using primer 3 software. The β-actin was used as the housekeeping gene. The real-time PCR program consisted of the following steps: 95 °C for 1 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 15 s, 72 °C for 45 s, and one step of 95 °C for 10 s. Melting curves were generated by increasing the temperature from 65 to 95 °C at a rate of 0.5 °C/s to denature the double-stranded DNA. The relative mRNA expression levels of genes were calculated using the comparative CT method (2−ΔΔCT). The values are presented as mean n-fold differences compared to the control (Livak and Schmittgen 2001).
Immune response parameters
Addition of hemolymph to anticoagulant solution using Neubauer chamber was used to determine total hemocyte counts (THCs). The anticoagulant solution was made up of 1:2, 0.45 M NaCl, 0.1 M glucose, 30 mM sodium citrate, 26 mM citric acid, and 10 mM EDTA at pH 4.6 (Söderhäll and Smith 1983). The method of Bradford (1976) was used to measure the total protein concentration in the sera while bovine serum albumin served as the protein standard. The lysozyme (LYZ) activity was determined using a spectrophotometer with a lysozyme-detection kit according to Ellis (1990). The phenoloxidase (PO) activity was determined spectrophotometrically (490 nm) according to Perazzolo and Barracco (1997). The phagocytic activity was assayed in accordance with the procedures of Selvin et al. (2004).
Bacterial challenge
After the feeding experiment, the V. harveyi bacterium was provided by Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, Alexandria University, Egypt. The V. harveyi isolates were previously isolated from naturally diseased shrimp and identified by traditional methods as well as molecular analysis. V. harveyi isolate was grown on tryptic soy broth (TSB with 1.5% NaCl, pH 7.5 at 30 °C for 48 h) (Joshi et al. 2014).
Fifteen (15) shrimps (V. harveyi free) from each tank were then allotted to a new 15-L water glass tanks (three tanks per treatment). V. harveyi was added into each tank to obtain a final concentration of 1 × 105 CFU/mL for 2 h (Robertson et al. 1998). During the challenge trials, animals continued to be fed on their corresponding experimental diets as in the feeding experiment four times per day for additional 10 days (Robertson et al. 1998). Salinity, dissolved oxygen, and water temperature were maintained at the same levels as in the feeding experiment. Leftover feed and feces were siphoned off every 2 days. The mortality and any abnormal signs of animals were recorded. The relative percent of survival (RPS) was calculated in the 10-day post-challenge (Amend 1981) as follows:
RPS = 100 [1 − (% mortality in treated fish/% mortality in the control fish)].
Statistical analysis
One-way analysis of variance was used to analyze the data obtained and to determine any significant differences among shrimp fed graded levels of dietary AX. The polynomial contrast procedure was employed to test the polynomial trend or relationship (linear or quadratic response) that exists between pairs of means in the presence of a significant interaction effect. This method enabled statistical evaluation of whether the observed effects were linear, in other words, directly proportional to the graded levels of supplemental AX, or quadratic (deviating from linearity), in other words, dose-dependent. Differences among means were deemed as statistically significant at P < 0.05 using the Duncan test as a post hoc test. All data were analyzed with SPSS software version 20 for Windows according to Dytham (2011).
Results
Antibacterial activity of astaxanthin against V. harveyi.
The growth of V. harveyi was prevented by adding AX to its culture medium, indicating the antibacterial activity of AX against V. harveyi (Fig. 1).
Growth indices
The growth performance and feed utilization of white-leg shrimp (L. vannamei) fed with different levels of AX for 8 weeks are shown in Table 3. The growth indices (final weight, % weight gain, and specific growth rate) of the control group were significantly lower than those of the AX-fed animal groups. Among the AX-fed groups, the growth indices from the 100- and 200-mg AX/kg feed treatments did not differ significantly (P > 0.05). Similarly, all the AX-fed shrimp consumed more feed than the control group, and we observed no significant (P > 0.05) difference between the 100- and 200-mg AX/kg feed treatments. FCR values ranged from 1.68 to 1.82, and we observed no significant (P > 0.05) differences among different AX treatments. Shrimp survival during the experimental period ranged from 83.3 to 87.7% with no significant (P > 0.05) differences among different treatments (Table 3).
Antioxidant activity and lipid peroxidation
The antioxidant enzyme activities in sera of L. vannamei fed on the experimental diets are presented in Table 4. Compared to the control group, SOD, CAT, and GPx activities, as well as GSH levels, were significantly (P < 0.05) higher in L. vannamei fed with diets enriched with AX up to 100 mg/kg feed, with no significant (P > 0.05) difference observed between 100- and 200-mg AX/kg feed treatments (Table 4). Similarly, shrimp fed with diets supplemented with AX up to 200-mg/kg diet had TAC levels that were significantly (P < 0.05) higher than those of the control. In contrast, the MDA levels of shrimp fed with AX-enriched diets were significantly (P < 0.05) lower than that of those fed with the control diet, which had the highest MDA level (Table 4).
The mRNA expression levels of cMn-SOD, CAT, and GPx in the hepatopancreas of white-leg shrimp were both linearly and quadratically (P < 0.05) upregulated in response to 8 weeks of the dietary supplementation of AX (Fig. 2). The maximum expression levels of these genes were observed in the 200-mg AX/kg feed treatment. In the case of GPx, the expression levels in the 100- and 200-mg AX/kg feed treatments did not differ significantly (P > 0.05; Fig. 2).
Intestinal morphometry
The results of morphometric analysis of the gastrointestinal tract of L. vannamei fed with different levels of AX are shown in Fig. 3. Compared to the control group, villi length, villi width, and absorption area were significantly increased in shrimp supplemented with AX for 8 weeks (Fig. 3). Moreover, the intestinal villi and its associated crypt, tunica muscularis (internal and external muscular layers), and submucosal tissues were free of inflammatory and/or degenerative changes in AX-fed shrimp. These shrimp also displayed properly arranged columnar epithelia and goblet cells. Interestingly, the thickness of both layers of tunica muscularis in the control and AX-fed groups did not differ significantly.
Immunological parameters
Feeding L. vannamei with distinct AX-containing diets was associated with significant linear (P < 0.05) and quadratic (P < 0.05) increases in the THC, total protein (TP), LYZ, phagocytic activity (PA), and phenoloxidase (PO) values, and the highest values were recorded in the 200-mg AX/kg diet (Table 5). Animals fed with the control diet exhibited significantly (P < 0.05) lower THC, TP, LYZ, PA, and PO values.
Bacterial challenge
Significant effects of dietary AX on the survival of L. vannamei after the exposure to V. harveyi were observed. Animals fed the 25-, 50-, 100-, and 200-mg AX/kg diet exhibited significant increases in their survival rate compared to those fed the control diet (Fig. 4). The dietary AX significantly increased (P < 0.05) the relative percentage of survival of L. vannamei, especially at the treatment of 200-mg/kg diet (82.7%).
Discussion
Growth indices
Astaxanthin has attracted wide attention because of its various physiological functions in aquatic animals (Lim et al. 2018; Lu et al. 2021). Here, feeding AX-enriched diets to L. vannamei significantly enhanced its growth characteristics and feed intake than the control group, where the highest shrimp growth taking place in the 100–200-mg AX/kg feed treatments. These results can be attributed to the influence of dietary AX on intestinal morphometry, specifically, to the expanded gut surface area (reflected by increased villi width, villi height, and absorption area) that resulted in improved nutrient absorption (Figs. 3 and 4). Furthermore, addition of AX to shrimps feed resulted in the increased secretion of digestive enzymes resulting in increased digestion, absorption, and utilization of nutrients, leading to enhanced feed intake, as we found in the current study. In a similar study, Zhang et al. (2013) showed that L. vannamei fed with diets containing 0-, 25-, 50-, 75-, 100-, 125-, and 150-mg AX/kg feed for 56 days resulted in significantly improved growth performance with optimum AX levels of 125–150-mg/kg diet. In another study, Wang et al. (2018) fed juvenile kuruma shrimp (Marsupenaeus japonicus) diets with 0-, 200-, 400-, 800-, 1200-, and 1600-mg AX/kg feed for 56 days, resulting in better growth performance in the AX-fed animals compared to the control group, with the best performance exhibited by shrimp fed the diet with 400-mg AX/kg feed. Wang et al. (2020) reported that L. vannamei fed with diets containing 0-, 50-, and 100-mg AX/kg feed for 4 weeks resulted in significantly higher weight gain and SGR values in the AX-fed animals compared to the control group.
Antioxidant activity and lipid peroxidation
Development of defense mechanisms by various organisms have helped them in neutralizing the harmful effects of reactive oxygen species (ROS; Lesser 2006; Hoseinifar et al. 2021). These mechanisms are in forms of enzymatic and non-enzymatic antioxidants of endogenous and exogenous origins. Structurally, AX is particularly interesting as a non-enzymatic antioxidant because it is able to act by reducing singlet oxygen and scavenging free radicals to obstruct chain reactions, and thereby thwarting oxidative damage to cellular macromolecules (Hussein et al. 2006; Liu and Osawa 2007).
Usually, the best option for evaluating the oxidative stress status is by measuring the levels or activities of several biomarkers. Here, feeding L. vannamei with AX-supplemented diets resulted in significantly increased activities of SOD, CAT, and GPX and increased levels of GSH and TAC. Furthermore, dietary AX significantly upregulated the expression of cMN-SOD, CAT, and GPx genes. These findings suggest that dietary AX improves the capacity of the shrimp’s antioxidant system, ultimately protecting L. vannamei from oxidative damage. Due to their molecular structure, carotenoid pigments, including AX, have several positive effects on biological systems, and these are directly linked to these pigments’ antioxidant properties and their very high scavenging affinity for toxic oxygen radicals (Ambati et al. 2014). Yang et al. (2010) found that feeding white shrimp with carotenoid-rich red yeast (Rhodosporidium paludigenum) resulted in significant increases in SOD, CAT, and GPx activities. In contrast, Zhang et al. (2013) reported that increasing dietary AX levels significantly reduces SOD and CAT activities, which is accompanied by the downregulation of cMn-SOD and CAT genes in L. vannamei. Wang et al. (2015) suggested that dietary AX promotes the expression of antioxidant-related mRNA for enzymes in the hepatopancreas of L. vannamei. Rahman et al. (2016) reported that rainbow trout AX-containing diets upregulated the antioxidant activity of rainbow trout compared to that fed with no AX.
The byproduct of unsaturated fatty acid peroxidation is MDA, which is commonly evaluated by TBARS analysis (Lin and Shiau 2005). It is known that the accumulation of MDA in cells depends on the level of ROS scavenging properties in an inverse manner. Here, L. vannamei fed with AX-supplemented diets had significantly lower MDA levels compared to the control group, which may be because the dietary AX is susceptible to ROS (as are many carotenoids) due to the presence of conjugated double bonds in their molecules. Thus, the MDA levels of shrimp (Penaeus monodon) fed with β-carotene and AX-supplemented diets were significantly lower than those of shrimp fed with the control diet (without carotenoids) (Niu et al. 2014). Similar results have been observed after feeding white-leg shrimp and rainbow trout diets that contained red yeast (Yang et al. 2010) and lycopene (Sahin et al. 2014), respectively.
Intestinal morphometry
Most of the nutrient absorption is taking place in the animals’ intestine. Here, nutrients are transported into and out of the intestinal enterocytes through specific transporters located at the brush-border and basolateral membranes (Nicholson et al. 2012; Abdel-Tawwab et al. 2018, 2021; Adeshina et al. 2019; Abdel-Latif et al. 2020). Histomorphometric analysis of the intestine of all experimental groups in the present study showed no inflammation in the intestinal tissues. Indeed, dietary AX significantly enhanced the villi length, villi width, and the absorption area of the gastrointestinal tract of L. vannamei in the order of the dosages. These findings suggest that dietary AX is beneficial for gut health and nutrient absorption, which have positive impacts on the growth of L. vannamei. However, long villi are usually associated a healthy gut and efficient nutrient absorption, resulting in improved the growth performance (Sklan et al. 2004; Trushenski 2015; Huerta-Aguirre et al. 2019). Shrimps’ growth is positively related to villus height/width ratio and absorption area because these properties increase nutrient uptake in the gut, which improves the feed utilization and growth performance of numerous fish species (Abdel-Tawwab et al. 2018, 2021; Adeshina et al. 2019; Abdel-Latif et al. 2020).
Immune responses
Serum proteins are vital elements for sustaining a healthy immune system in aquatic animals (Shiu et. al. 2017; Lim et al. 2021; Abdel-Tawwab et al. 2022). The results of this study reveal significant increase in the serum TP of AX-fed animals, which are indicative of rising antibody production and an enhanced immune response. The raised serum TP may also assist in repairing damaged tissues and eliminating bacteria (Gerwich et al. 2002; Laith et al. 2017). These results are consistent with those of Jagruthi et al. (2014) and Lim et al. (2021), who reported that dietary AX was capable of increasing serum TP levels in common carp and Asian seabass, respectively.
Innate immunity is the pivotal defense mechanism against invading pathogenic organisms (Romo et al. 2016). Phagocytes provide innate immunity through engulfing, terminating, and digesting foreign microbes (Abarike et al. 2018). LYZ is a bacteriolytic component of the immune system that catalyzes the hydrolysis of the bacterial cell wall, in addition to increasing the capacity for activating the complement system (Magnadóttir 2006; Ragland and Criss 2017). Phagocytic cells are the primary producers of LYZ (Grayfer et al. 2018). The secretion of LYZ by phagocytes is a marker of PA, whereby both its secretion and the activity increase simultaneously during the onset and progress of an infection. Diets supplemented with AX considerably improved phagocytic and serum LYZ activities, which directly enhanced the resistance of the shrimp to vibriosis. A markedly lower secretion and decreased activity indicate immune suppression in the control shrimp caused by the infection, presumably due to an absence of the stimulatory effect of dietary AX. The intensification of phagocytosis and serum LYZ activity could be associated with the higher THC count of the AX-fed animals. These results are consistent with those of previous studies (Ali et al. 2018; Lim et al. 2019a, b; Lim et al. 2021). The enhancement of phagocytic and serum LYZ activities in shrimp in this study might be also related to macrophage activation and stimulation of other humoral factors (Tang et al. 2010; Laith et al. 2017), resulted from dietary AX supplementation. Similar results were reported by Jagruthi et al. (2014), Li et al. (2020), and Lim et al. (2021), who revealed that dietary AX was capable of enhancing phagocytic and serum LYZ activities in common carp, snakehead (Channa argus), and Asian seabass, respectively.
Antibacterial activity
The ultimate goal of providing L. vannamei with immunostimulating diets is to improve its protection against infectious bacteria and thus its overall disease resistance. This study demonstrated that feeding L. vannamei with AX-enriched diets allowed effectively controlling the occurrence of V. harveyi infection. This result is attributed to the antibacterial activity that was demonstrated in this study (Fig. 1). The higher survival and disease tolerance of AX-fed shrimp against pathogenic V. harveyi are the outcome of the complex interactions between enhanced biochemical and innate immune defense mechanisms. In addition, dietary AX may have activated the antioxidant response mechanism for optimum cellular functions and physiological improvements (Xie et al. 2017; Lim et al. 2019a, b). The beneficial effects of dietary AX from various sources on the survival of aquatic animals have been widely reported (Jagruthi et al. 2014; Liu et al. 2016; Lim et al. 2019a, b; Lim et al. 2021). In a similar study, Jagruthi et al. (2014) fed common carp (Cyprinus carpio) with 0-, 25-, 50-, and 100-mg AX/kg diet and investigated the immune response and disease resistance against Aeromonas hydrophila infection. Wang et al. (2015) fed L. vannamei with 0.0- and 80-mg AX/kg diet for 4 weeks and evaluated their effects on the immune response and resistance to the white spot syndrome virus. Their results revealed that the mortality of the AX-fed group 11 days after infection was 76.3%, whereas that of the control group was 100%. Xie et al. (2018) reported that dietary AX increased the survival of L. vannamei and its tolerance to V. parahaemolyticus infection. Lim et al. (2021) fed Asian seabass with 0.0-, 50-, 100-, and 150-mg AX/kg diet for 90 days and artificially infected with V. alginolyticus at the end of the feeding period. They reported that supplementary feeding of AX was effective in reinforcing fish immunity and disease resistance against V. alginolyticus infection. Another study reported that when albino Oscar fish (Astronotus ocellatus) infected with pathogenic Aeromonas hydrophila were fed with 0.0- and 200-mg AX/kg diet, the cumulative mortality reached 76.7% and 56.7%, respectively (Alishahi et al. 2015). In addition, the increase of disease resistance by feeding fish with AX was also observed in olive flounders (Paralichthys olivaceus) infected with Edwardsiella tarda (Kim et al. 2012). Thus, the improvement of disease resistance and increase of survival in white-leg shrimp (L. vannamei) by feeding AX-enriched diets appears as a promising tool for more eco-friendly shrimp aquaculture.
Conclusions
We have shown that the inclusion of AX to diets for white-leg shrimp results in improved growth indices, feed utilization, and intestinal histomorphometry. The results obtained indicate that dietary AX at levels of 100–200-mg/kg diet acts as a natural antioxidant that can enhance the antioxidant status of white-leg shrimp via modulating the antioxidant-related genes along with reducing the levels of lipid peroxidation. Additionally, the dietary AX modulated the immunity and promoted the protection of white-leg shrimp (L. vannamei) against V. harveyi infection. These results suggest the effectiveness of using dietary AX in aquafeeds at levels of 100-mg/kg diet to enhance the growth, antioxidant status, and immune response of white-leg shrimp and increase its resistance to possible bacterial infections.
Data availability
Data of the present article are available under request.
Code availability
Not applicable.
References
Abarike ED, Kuebutornye FKA, Jian J, Tang J, Lu Y, Cai J (2018) Influences of immunostimulants on phagocytes in cultured fish. Rev Aquacult 11:1219–1227
Abdel-Latif HM, Abdel-Tawwab M, Khafaga AF, Dawood MA (2020) Dietary oregano essential oil improved the growth performance via enhancing the intestinal morphometry and hepato-renal functions of common carp (Cyprinus carpio L.) figerlings. Aquaculture 526:735432
Abdel-Tawwab M, Adeshina I, Jenyo-Oni A, Ajani EK, Emikpe BO (2018) Growth, physiological, antioxidants, and immune response of African catfish, Clarias gariepinus (B.), to dietary clove basil, Ocimum gratissimum, leaf extract and its susceptibility to Listeria monocytogenes infection. Fish Shellfish Immunol 78:346–354
Abdel-Tawwab M, Shukry M, Farrag FA, El-Shafai NM, Dawood MAO, Abdel-Latif HMR (2021) Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 536:736467
Abdel-Tawwab M, Abdel-Razek N, Tahoun A, Awad SMM, El-Ashram AM (2022) Effects of dietary supplementation of chamomile oil on Indian shrimp (Penaeus indicus) performance, antioxidant, innate immunity, and resistance to Vibrio parahaemolyticus infection. Aquaculture 552:738045
Adel M, Dadar M, Oliveri Conti G (2017) Antibiotics and malachite green residues in farmed rainbow trout (Oncorhynchus mykiss) from the Iranian markets: a risk assessment. Int J Food Prop 20:402–408
Adeshina I, Jenyo-Oni A, Emikpe BO, Ajani EK, Abdel-Tawwab M (2019) Stimulatory effect of dietary clove, Eugenia caryophyllata, bud extract on growth performance, nutrient utilization, antioxidant capacity, and tolerance of African catfish, Clarias gariepinus (B.), to Aeromonas hydrophila infection. J World Aquacult Soc 50:390–405
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alagawany M, Farag MR, Salah AS, Mahmoud MA (2020) The role of oregano herb and its derivatives as immunomodulators in fish. Rev Aquac 12:2481–2492
Ali M, Soltanian S, Akbary P, Gholamhosseini A (2018) Growth performance and lysozyme activity of rainbow trout fingerlings fed with vitamin E and selenium, marjoram (Origanum spp.), and ajwain (Trachyspermum ammi) extracts. J Appl Anim Res 46:650–660
Alishahi M, Karamifar M, Mesbah M (2015) Effects of astaxanthin and Dunaliella salina on skin carotenoids, growth performance and immune response of Astronotus ocellatus. Aquac Int 23:1239–1248
Ambati RR, Moi PS, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs 12:128–152
Amend DF (1981) Potency testing of fish vaccines. Fish Biologics: Serodiagnostics Vaccines 49:447–454
APHA (1998) Standard methods for the examination of the water and wastewater, 22nd edn. American Public Health Association, Washington, DC
Austin B, Zhang XH (2006) Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett Appl Microbiol 43:119–124
Benzie IF, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 7(72):248–254
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. In: Armstrong D (ed) Methods in Molecular Biology. Humana Press, Totowa, NJ, Free Radical and Antioxidant Protocols, pp 347–354
Chew BP (1995) Antioxidant vitamins affect food animal immunity and health. J Nut 125:1804–1808
Dawood MAO, Koshio S, Esteban MA (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquac 10:950–974
Dawood MA, Abdel-Tawwab M, Abdel-Latif HM (2020) Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in fish. Rev Aquac 12:2511–2526
Dytham C (2011) Choosing and using statistics: a biologist’s guide. Blackwell Science, London, UK
Ellis AE (1990) Lysozyme assay. In: Stolen JS, Fletcher DP, Anderson BS, Robertson BS (eds) Techniques in fish immunology. SOS Publication, Fair Haven, NJ, USA, pp 101–103
FAO (2020) The state of world fisheries and aquaculture 2020 — sustainability in action. Food Agric Org U N (FAO), Rome. Italy
Flohé L, Günzler WA (1984) Assay of glutathione peroxidase. Methods Enzymol 105:115–121
Gerwich L, Steinhauer R, Lapatra S, Sandell T, Ortuno J, Hajiseyedjavadi N (2002) The acute phase response of rainbow trout (Oncorhynchus mykiss) plasma protein to viral, bacterial and fungal inflammatory agents. Fish Shellfish Immunol 12:242–299
Grayfer L, Kerimoglu B, Yaparla A, Hodgkinson JW, Xie J, Belosevic M (2018) Mechanisms of fish macrophage antimicrobial immunity. Front Immunol 9:1105. https://doi.org/10.3389/fimu.2018.01105
Ho YH, Leung HM, Yuen SY, Ng KS, Li TS, Yuen LM et al (2018) Maximization of astaxanthin production from green microalga Haematococcus pluvialis using internally-illuminated photobioreactor. Adv Biosci Bioeng 6:10–22
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2021) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29:198–217
Huerta-Aguirre G, Paredes-Ramos KM, Becerra-Amezcua MP, Hernández-Calderas I, Matadamas-Guzman M, Guzmán-García X (2019) Histopathological analysis of the intestine from Mugil cephalus on environment reference sites. In: Pollut Water Bodies Latin Am Springer, 319 –328
Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H (2006) Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod 69:443–449
Jagruthi C, Yogeshwari G, Anbazahan SM, Mari LS et al (2014) Effect of dietary astaxanthin against Aeromonas hydrophila infection in common carp. Cyprinus Carpio Fish Shellfish Immunol 41:674–680
Jin J, Wang Y, Yao M, Gu X, Li B, Liu H et al (2018) Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol Biofuels 11:230
Joshi J, Srisala J, Truong VH, Chen I, Nuangsaeng B, Suthienkul O, Lo CF, Flegel TW, Sritunyalucksana K, Thitamadee S (2014) Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND). Aquaculture 428–429:297–302
Kim SS, Song JW, Kim KW, Lee KJ (2012) Effects of dietary astaxanthin on innate immunity and disease resistance against Edwardsiella tarda in olive flunder Paralichthys olivaceus. Isr J Aquac-Bamid 64:740
Laganà P, Caruso G, Minutoli E, Zaccone R, Delia S (2011) Susceptibility to antibiotics of Vibrio spp. and Photobacterium damsela ssp. piscicida strains isolated from Italian aquaculture farms. New Microbiol 34:53–63
Laith AA, Mazlan AG, Effendy AW, Ambak MA, Nurhafiah WWI, Alia AS et al (2017) Effect of Excoecaria agallocha on non-specific immune responses and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Res Vet Sci 112:192–200
Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Ann Rev Physiol 68:253–278
Li M-Y, Guo W-Q, Guo G-L, Zhu X-M, Niu X-T et al (2020) Effects of dietary astaxanthin on lipopolysaccharide-induced oxidative stress, immune responses and glucocorticoid receptor (GR)-related gene expression in Channa argus. Aquaculture 517:734816
Lim KC, Yusoff FM, Shariff M, Kamarudin MS (2018) Astaxanthin as feed supplement in aquatic animals. Rev Aquacult 10:738–773
Lim KC, Yusoff FM, Shariff M, Kamarudin MS, Nagao N (2019) Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 512:734339
Lim KC, Yusoff FM, Shariff M, Kamarudin MS (2019) Dietary administration of astaxanthin improves feed utilization, growth performance and survival of Asian seabass, Lates calcarifer (Bloch, 1790). Aquac Nutr 25:1410–1421
Lim KC, Yusoff FM, Shariff M, Kamarudin MS (2021) Dietary astaxanthin augments disease resistance of Asian seabass, Lates calcarifer (Bloch, 1790), against Vibrio alginolyticus infection. Fish Shellfish Immunol 114:90–101
Lin YH, Shiau SY (2005) Dietary vitamin E requirement of grouper, Epinephelus malabaricus, at two lipid levels, and their effects on immune responses. Aquaculture 248:235–244
Liu X, Osawa T (2007) Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem Biophys Res Commun 357:187–193
Liu F, Shi HZ, Guo QS, Yu YB, Wang AM, Lv F et al (2016) Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol 51:125–135
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2-DDCT method. Methods 25:402–408
Lu Q, Huankai Li H, Zou Y, Liu H, Yang L (2021) Astaxanthin as a microalgal metabolite for aquaculture: a review on the synthetic mechanisms, production techniques, and practical application. Algal Research 54:102178
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunology 20:137–151
Maron DF, Smith TJS, Nachman KE (2013) Restrictions on anti-microbial use in food animal production: an international regulatory and economic survey. Glob Health 9(1):48. https://doi.org/10.1186/1744-8603-9-48
McCord JM, Fridovich I (1969) Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) Host-gut microbiota metabolic interactions. Science 336(6086):1262–1267
Niu J, Wen H, Li C-H, Liu Y-J, Tian L-X, Chen X, Huang Z, Lin H-Z (2014) Comparison effect of dietary astaxanthin and β-carotene in the presence and absence of cholesterol supplementation on growth performance, antioxidant capacity and gene expression of Penaeus monodon under normoxia and hypoxia condition. Aquaculture 422:8–17
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides. In: Animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Pashkow FJ, Watumull DG, Campbell CL (2008) Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am J Cardiol 101:58–68
Perazzolo LM, Barracco MA (1997) The prophenoloxidase activating system of the shrimp Penaeus paulensis and associated factors. Dev Comp Immunol 21(5):385–395
Ragland SA, Criss AK (2017) From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLoS Pathog 13(2017):e1006512. https://doi.org/10.1371/journal.ppat.1006512
Rahman M, Khosravi S, Chang KH, Lee S (2016) Effects of dietary inclusion of astaxanthin on growth, muscle pigmentation and antioxidant capacity of juvenile rainbow trout (Oncorhynchus mykiss). Prev Nutr Food Sci 21:281–288
Roberts RJ, Smail DA (2001) Laboratory methods. In: Roberts RJ (ed) Fish pathology, 3rd edn. Saunders, London, W.B, pp 380–412
Robertson PAW, Calderon J, Carrera F, Stark JR, Zherdmant M, Austin B (1998) Experimental Vibrio harveyi infections in Penaeus vannamei larvae. Dis Aquat Org 32:151–155
Romo MR, Perez-Martinez D, Ferrer CC (2016) Innate immunity in vertebrates: an overview. Immunology 148:125–139
Sahin K, Yazlak H, Orhan C, Tuzcu M, Akdemir F, Sahin N (2014) The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture 418:132–138
Santos L, Ramos F (2018) Antimicrobial resistance in aquaculture: current knowledge and alternatives to tackle the problem. Int J Antimicrob Agents 52:135–143
Selvin J, Huxley AJ, Lipton AP (2004) Immunomodulatory potential of marine secondary metabolites against bacterial diseases of shrimp. Aquaculture 230:241–248
Shiu YL, Chiu KH, Huynh TG, Liu PC, Liu CH (2017) Plasma immune protein analysis in the orange-spotted grouper Epinephelus coioides: evidence for altered expressions of immune factors associated with a choline-supplemented diet. Fish Shellfish Immunol 65:235–243
Sklan D, Prag T, Lupatsch I (2004) Structure and function of the small intestine of the tilapia Oreochromis niloticus× Oreochromis aureus (Teleostei, Cichlidae). Aquac Res 35:350–357
Söderhäll K, Smith VJ (1983) Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution. Dev Comp Immunol 7(2):229–239
Tang F, Zhan W, Sheng X, Chi H (2010) Immune response of Japanese flunder Paralichthys olivaceus to outer membrane protein of Edwardsiella tarda. Fish Shellfish Immunol 28:333–343
Teo JWP, Suwanto A, Poh CL (2000) Novel ß-lactamase genes from two environmental isolates of Vibrio harveyi. Antimicrob Agents Chemother 44:1309–1314
Trushenski J (2015) 8 - Nutritional impacts on fish mucosa: dietary considerations. Mucosal Health Aquac 199–209
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96
Wang H, Dai A, Liu F, Guan Y (2015) Effects of dietary astaxanthin on the immune response, resistance to white spot syndrome virus and transcription of antioxidant enzyme genes in Pacific white shrimp Litopenaeus vannamei. Iran J Fish Sci 14:699–718
Wang W, Ishikawa M, Koshio S, Yokoyama S, Sakhawat Hossain M, Moss AS (2018) Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture 491:197–204
Wang Y, Wang B, Liu M, Jiang K, Wang M, Wang L (2020) Comparative transcriptome analysis reveals the potential influencing mechanism of dietary astaxanthin on growth and metabolism in Litopenaeus vannamei. Aquaculture Reports 16:100259
Xie J, Chen X, Niu J, Wang J, Wang Y, Liu Q (2017) Effects of astaxanthin on antioxidant capacity of golden pompano (Trachinotus ovatus) in vivo and in vitro. Fish Aquat Sci 20:1–8
Xie SW, Fang WP, Wei D, Liu YJ, Yin P, Niu J, Tian LX (2018) Dietary supplementation of Haematococcus pluvialis improved the immune capacity and low salinity tolerance ability of post-larval white shrimp. Litopenaeus Vannamei Fish Shellfish Immunol 80:452–457
Yang S-P, Wu Z-H, Jian J-C, Zhang X-Z (2010) Effect of marine red yeast Rhodosporidium paludigenum on growth and antioxidant competence of Litopenaeus vannamei. Aquaculture 309:62–65
Yano Y, Hamano K, Satomi M, Tsutsui I, Ban M, Aue-umneoy D (2014) Prevalence and antimicrobial susceptibility of Vibrio species related to food safety isolated from shrimp cultured at inland ponds in Thailand. Food Control 38:30–36
Yilmaz S, Yilmaz E, Dawood MAO, Ringø E, Ahmadifar E, Abdel-Latif HMR (2022) Probiotics, prebiotics, and symbiotics used to control vibriosis in fish: a review. Aquaculture 737514
Zhang J, Liu YJ, Tian LX, Yang HJ, Liang GY, Yue YR, Xu DH (2013) Effects of dietary astaxanthin on growth, antioxidant capacity and gene expression in Pacific white shrimp Litopenaeus vannamei. Aquac Nutr 19:917–927
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Elsayed A. A. Eldessouki: conceptualization, investigation, methodology; Amany M. Diab: conceptualization, investigation, methodology; Talal A. M. Abo Selema: conceptualization, investigation, methodology; Nader M. Sabry: conceptualization, investigation, methodology; Mahmoud M. Abotaleb: conceptualization, investigation, methodology; Riad H. Khalil: conceptualization, investigation, methodology; Nasser El-Sabbagh: conceptualization, investigation, methodology; Nehal A. Younis: conceptualization, investigation, methodology; Mohsen Abdel-Tawwab: conceptualization, supervision, data curation, writing — original draft, writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of fish were followed by the authors.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eldessouki, E.A.A., Diab, A.M., Selema, T.A.M.A. et al. Dietary astaxanthin modulated the performance, gastrointestinal histology, and antioxidant and immune responses and enhanced the resistance of Litopenaeus vannamei against Vibrio harveyi infection. Aquacult Int 30, 1869–1887 (2022). https://doi.org/10.1007/s10499-022-00876-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00876-w