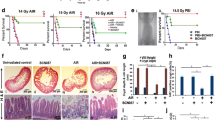
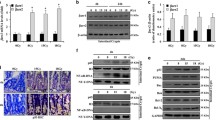
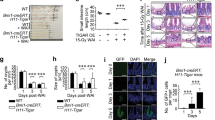
Abstract
It has been well established that radiation-induced gastrointestinal injury is manifested through loss of intestinal crypt stem cells and disruption of the mucosal layers, resulting in diarrhoea, weight loss, electrolyte imbalance, infection and mortality. Podophyllotoxin and rutin in combination (G-003M) has been reported to regulate endogenous cellular antioxidant defense systems and inflammatory response. However, the mechanism by which G-003M ameliorates radiation-induced intestinal stem cell (ISC) injury remains unclear. Here, we hypothesize the radioprotective potential of G-003M would amplify the intestinal crypt stem cells through upregulation of Wnt/β-catenin signaling and accelerate the reconstitution of the irradiated intestine. Our results showed significant functional and structural intestine regeneration in irradiated animals following G-003M treatment which resulted in improved animal survival. Immunohistochemical examination revealed an enhancement in Lgr5+ ve crypt stem cells. Increased β-catenin nuclear translocation resulted in upregulation of β-catenin target genes that supported ISC renewal and expansion in G-003M-treated mice, as compared to IR-treated mice. However, G-003M could not rescue the Wnt knockdown cohorts (XAV939 treated) which exhibited greater incidence of intestinal apoptosis, DNA damage and crypt depopulation upon radiation exposure. These findings suggest the involvement of Wnt pathway during G-003M mediated amelioration of IR-induced ISC injury. G-003M also minimised acute inflammation by restricting the infiltration of immune cells into the intestinal venules. Furthermore, G-003M treated animals showed improved anti-tumor response compared to FDA approved Amifostine. Taken together, our findings suggest that G-003M may be used as a potential countermeasure for radiation injuries as well as an adjuvant during anti-cancer therapy.





Similar content being viewed by others
References
Barker N, Van Es JH, Kuipers J, Kujala P, Van Den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ (2007) Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449(7165):1003
Zhu L, Gibson P, Currle DS, Tong Y, Richardson RJ, Bayazitov IT, Poppleton H, Zakharenko S, Ellison DW, Gilbertson RJ (2009) Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature 457(7229):603
Sangiorgi E, Capecchi MR (2008) Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40(7):915
Potten CS, Kovacs L, Hamilton E (1974) Continuous labelling studies on mouse skin and intestine. Cell Prolif 7(3):271–283
Cheng H, Leblond C (1974) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine III. Entero-endocrine cells. Am J Anat 141(4):503–519
Monti P, Wysocki J, Van der Meeren A, Griffiths N (2005) The contribution of radiation-induced injury to the gastrointestinal tract in the development of multi-organ dysfunction syndrome or failure. Br J Radiol (1):89–94
Gudkov AV, Komarova EA (2010) Pathologies associated with the p53 response. Cold Spring Harb Perspect Biol 2(7):a001180
Gregorieff A, Clevers H (2005) Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev 19(8):877–890
Leedham S, Brittan M, McDonald S, Wright N (2005) Intestinal stem cells. J Cell Mol Med 9(1):11–24
Salin C, Samanta N, Goel H (2001) Protection of mouse jejunum against lethal irradiation by Podophyllum hexandrum. Phytomedicine 8(6):413–422
Bump E, Malaker K (1998) Radioprotectors: Chemical. biological and clinical perspective. CRC Press, Boca Raton
Nair CK, Parida DK, Nomura T (2001) Radioprotectors in radiotherapy. J Radiat Res 42(1):21–37
De Souza C, Santini G, Marino G, Nati S, Congiu A, Vigorito A, Damasio E (2000) Amifostine (WR-2721), a cytoprotective agent during high-dose cyclophosphamide treatment of non-Hodgkin’s lymphomas: a phase II study. Braz J Med Biol Res 33(7):791–798
Hosseinimehr SJ (2007) Trends in the development of radioprotective agents. Drug Discov Today 12(19–20):794–805
Maisin J-R (1998) Bacq and Alexander award lecture chemical radioprotection: past, present and future prospects. Int J Radiat Biol 73(4):443–450
Weiss JF, Landauer MR (2003) Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 189(1–2):1–20
Jagetia GC (2007) Radioprotective potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr 40(2):74–81
Gupta M, Agrawala P, Kumar P, Devi M, Soni N, Tripathi R (2008) Modulation of gamma radiation-inflicted damage in Swiss albino mice by an alcoholic fraction of Podophyllum hexandrum rhizome. J Med Food 11(3):486–492
Sankhwar S, Gupta ML, Gupta V, Verma S, Suri KA, Devi M, Sharma P, Khan EA, Alam MS (2011) Podophyllum hexandrum-mediated survival protection and restoration of other cellular injuries in lethally irradiated mice. Evidence-Based Complementary and Alternative Medicine. https://doi.org/10.1093/ecam/nep061
Lata M, Prasad J, Singh S, Kumar R, Singh L, Chaudhary P, Arora R, Chawla R, Tyagi S, Soni N (2009) Whole body protection against lethal ionizing radiation in mice by REC-2001: a semi-purified fraction of Podophyllum hexandrum. Phytomedicine 16(1):47–55
Kalita B, Ranjan R, Singh A, Yashavarddhan M, Bajaj S, Gupta ML (2016) A combination of podophyllotoxin and rutin attenuates radiation induced gastrointestinal injury by negatively regulating NF-κB/p53 signaling in lethally irradiated mice. PLoS ONE 11(12):e0168525
Verma S, Kalita B, Bajaj S, Prakash H, Singh AK, Gupta ML (2017) a combination of Podophyllotoxin and rutin alleviates radiation-induced Pneumonitis and Fibrosis through Modulation of lung inflammation in Mice. Front Immunol 8:658
Singh A, Yashavarddhan M, Kalita B, Ranjan R, Bajaj S, Prakash H, Gupta ML (2017) Podophyllotoxin and rutin modulates ionizing radiation-induced oxidative stress and apoptotic cell death in mice bone marrow and spleen. Front Immunol 8:183
Potten CS, Booth D, Haley J (1997) Pretreatment with transforming growth factor beta-3 protects small intestinal stem cells against radiation damage in vivo. Br J Cancer 75(10):1454
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME (2008) Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8(4):299
Manetti R, Parronchi P, Giudizi MG, Piccinni M, Maggi E, Trinchieri G, Romagnani S (1993) Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med 177(4):1199–1204
Seder RA, Gazzinelli R, Sher A, Paul WE (1993) Interleukin 12 acts directly on CD4 + T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci 90(21):10188–10192
Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu C-y, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK (1996) IL-12-deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity 4(5):471–481
Lu H, Ouyang W, Huang C (2006) Inflammation, a key event in cancer development. Mol Cancer Res 4(4):221–233
Qiu W, Wang X, Buchanan M, He K, Sharma R, Zhang L, Wang Q, Yu J (2013) ADAR1 is essential for intestinal homeostasis and stem cell maintenance. Cell Death Dis 4(4):e599
Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76(2):301–314
Hallahan D, Kuchibhotla J, Wyble C (1996) Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res 56(22):5150–5155
Mollà M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, Piqué JM, Panés J (2003) Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys 57(1):264–273
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu J-W, Meng R, Quong AA, Latz E, Scott CP (2010) The anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem. https://doi.org/10.1074/jbc.M109.082305
Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H (1998) Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet 19(4):379
Kuhnert F, Davis CR, Wang H-T, Chu P, Lee M, Yuan J, Nusse R, Kuo CJ (2004) Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci 101(1):266–271
Pinto D, Gregorieff A, Begthel H, Clevers H (2003) Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev 17(14):1709–1713
Tian X-H, Hou W-J, Fang Y, Fan J, Tong H, Bai S-L, Chen Q, Xu H, Li Y (2013) XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res 32(1):100
Kim PJ, Plescia J, Clevers H, Fearon ER, Altieri DC (2003) Survivin and molecular pathogenesis of colorectal cancer. The Lancet 362(9379):205–209
Zhang J, Wang X, Chen B, Xiao Z, Li W, Lu Y, Dai J (2010) The human pluripotency gene NANOG/NANOGP8 is expressed in gastric cancer and associated with tumor development. Oncol Lett 1(3):457–463
Bettess MD, Dubois N, Murphy MJ, Dubey C, Roger C, Robine S, Trumpp A (2005) c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol 25(17):7868–7878
Thotala D, Chetyrkin S, Hudson B, Hallahan D, Voziyan P, Yazlovitskaya E (2009) Pyridoxamine protects intestinal epithelium from ionizing radiation-induced apoptosis. Free Radic Biol Med 47(6):779–785
Lu X, Nurmemet D, Bolduc DL, Elliott TB, Kiang JG (2013) Radioprotective effects of oral 17-dimethylaminoethylamino-17-demethoxygeldanamycin in mice: bone marrow and small intestine. Cell Biosci 3(1):36
Uma Devi P, Ganasoundari A, Vrinda B, Srinivasan K, Unnikrishnan M (2000) Radiation protection by the ocimum flavonoids orientin and vicenin: mechanisms of action. Radiat Res 154(4):455–460
Jagetia GC, Baliga MS, Malagi K, Kamath MS (2002) The evaluation of the radioprotective effect of triphala (an ayurvedic rejuvenating drug) in the mice exposed to γ-radiation. Phytomedicine 9(2):99–108
Jagetia GC, Venkatesh P, Archana P, Krishnanand BR, Baliga MS (2006) Effects of Aegle marmelos (L.) Correa on the peripheral blood and small intestine of mice exposed to gamma radiation. J Environ Pathol Toxicol Oncol 25 (4)
Yuhas JM (1980) Active versus passive absorption kinetics as the basis for selective protection of normal tissues by S-2-(3-aminopropylamino)-ethylphosphorothioic acid. Cancer Res 40(5):1519–1524
Bhanja P, Saha S, Kabarriti R, Liu L, Roy-Chowdhury N, Roy-Chowdhury J, Sellers RS, Alfieri AA, Guha C (2009) Protective role of R-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice. PLoS ONE 4(11):e8014
Goel A, Aggarwal BB (2010) Curcumin, the golden spice from Indian saffron, is a chemosensitizer and radiosensitizer for tumors and chemoprotector and radioprotector for normal organs. Nutr Cancer 62(7):919–930
Lumniczky K, Sáfrány G (2015) The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett 356(1):114–125
Fedoročko P, Egyed A, Vacek A (2002) Irradiation induces increased production of haemopoietic and proinflammatory cytokines in the mice lung. Int J Radiat Biol 78(4):305–313
Frey B, Rubner Y, Wunderlich R, Weiss E-M, Pockley G, Fietkau A, R, S Gaipl U (2012) Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation-implications for cancer therapies. Curr Med Chem 19(12):1751–1764
Dutta A, Gupta M, Kalita B (2015) The combination of the active principles of Podophyllum hexandrum supports early recovery of the gastrointestinal system via activation of Nrf2-HO-1 signaling and the hematopoietic system, leading to effective whole-body survival in lethally irradiated mice. Free Radic Res 49(3):317–330
Singh VK, Romaine PL, Seed TM (2015) Medical countermeasures for radiation exposure and related injuries: characterization of medicines, FDA-approval status and inclusion into the strategic national stockpile. Health Phys 108(6):607
Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME (1997) Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem 272(34):21096–21103
Acknowledgements
We extend our sincere gratitude to Dr. Ajay Kumar Singh, Director, INMAS for providing necessary infrastructure and support to accomplish this work. We also thank Dr. B.G. Roy for providing experimental animals for the study. The support from Ms Anjali Sharma and Ms Namita Kalra for irradiation facility and flow cytometry measurements is duly acknowledged. BK, RR acknowledges UGC for the award of senior research fellowship.
Funding
This work was funded by the grant INM-313 from Defence Research & Development Organization (DRDO), Ministry of Defence, Govt. of India. BK, RR acknowledges University Grants Commission (UGC), Governtment of India for the award of senior research fellowship. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
BK MLG: Conceived, designed and performed the experiments. RR BK: Analyzed the data. RR BK: Contributed reagents/materials/analysis tool. MLG BK: Wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared no conflict of interest regarding the work presented in the manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalita, B., Ranjan, R. & Gupta, M.L. Combination treatment of podophyllotoxin and rutin promotes mouse Lgr5+ ve intestinal stem cells survival against lethal radiation injury through Wnt signaling. Apoptosis 24, 326–340 (2019). https://doi.org/10.1007/s10495-019-01519-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01519-x