Abstract
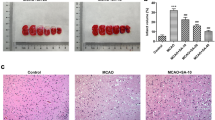
Reports have showed that Sigma-1 receptor (Sig-1R) activation can protect neurons against cerebral ischemia/reperfusion (I/R) injury in mice and alleviate endoplasmic reticulum (ER) stress in cultured cells, but little known is about the protective role of Sig-1R on ER stress induced by cerebral I/R. The purpose of this study was to determine whether Sig-1R exerts a protective effect against ER stress-mediated apoptosis in cerebral I/R using a 15-min bilateral common carotid artery occlusion (BCCAO) mouse model. At 72 h after reperfusion in BCCAO mice, we found that Sig-1R knockout (Sig-1R KO) significantly increased terminal dUTP nick-end labeling (TUNEL)-positive cells and nuclear structural damage in cortical neurons. Treatment with the Sig-1R agonist PRE084 once daily for three consecutive days reduced the number of TUNEL-positive cells and improved the ultrastructural damage of neurons in the cerebral cortex. These protective effects could be blocked by the Sig-1R antagonist BD1047. Then, we used BCCAO mice at 24 h after reperfusion to detect the expression of ER stress-mediated apoptotic pathway proteins. We found that expression of the pro-apoptotic proteins p-PERK, p-eIF2α, ATF, CHOP, p-IRE, p-JNK, Bim, PUMA, cleaved-caspase-12 and cleaved-caspase-3 was significantly increased and that expression of the anti-apoptotic protein Bcl-2 was significantly decreased in Sig-1R KO-BCCAO mice compared with BCCAO mice. Meanwhile, we found that treatment with PRE084 twice a day decreased pro-apoptotic protein expression and increased anti-apoptotic protein expression. The effects of PRE084 were blocked by the Sig-1R antagonist BD1047. These results suggest that Sig-1R activation inhibits ER stress-mediated apoptosis in BCCAO mice, indicating that Sig-1R may be a therapeutic target for neuroprotection particularly relevant to ER stress-induced apoptosis after cerebral I/R injury.








Similar content being viewed by others
References
Inagi R (2009) Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol 112(1):e1-9. https://doi.org/10.1159/000210573
Noh MR, Kim JI, Han SJ, Lee TJ, Park KM (2015) C/EBP homologous protein (CHOP) gene deficiency attenuates renal ischemia/reperfusion injury in mice. Biochim Biophys Acta 1852(9):1895–1901. https://doi.org/10.1016/j.bbadis.2015.06.004
Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL, Zhan J, Tong YN, Lin LR, He YN (2014) Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol 306(1):F75–F84. https://doi.org/10.1152/ajprenal.00117.2013
Cui Y, Zhao D, Barrow PA, Zhou X (2016) The endoplasmic reticulum stress response: a link with tuberculosis? Tuberculosis (Edinb) 97:52–56. https://doi.org/10.1016/j.tube.2015.12.009
Hoozemans JJ, Scheper W (2012) Endoplasmic reticulum: the unfolded protein response is tangled in neurodegeneration. Int J Biochem Cell Biol 44(8):1295–1298. https://doi.org/10.1016/j.biocel.2012.04.023
Nishitoh H (2004) Life and death under the ER stress condition. J Oral Biosci 46(4):259–269. https://doi.org/10.1016/s1349-0079(04)80015-x
Hayashi T, Su T (2005) The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol 3(4):267–280
Hayashi T, Su TP (2001) Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci USA 98(2):491–496. https://doi.org/10.1073/pnas.021413698
Hayashi T, Su TP (2004) Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs 18(5):269–284
Villard V, Espallergues J, Keller E, Alkam T, Nitta A, Yamada K, Nabeshima T, Vamvakides A, Maurice T (2009) Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid beta(25–35)-induced toxicity in mice. Neuropsychopharmacology 34(6):1552–1566. https://doi.org/10.1038/npp.2008.212
Phan VL, Urani A, Sandillon F, Privat A, Maurice T (2003) Preserved sigma1 (sigma1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol Aging 24(6):865–881. doi:S0197458002002312 [pii]
Takahashi H, Kirsch JR, Hashimoto K, London ED, Koehler RC, Traystman RJ (1996) PPBP [4-phenyl-1-(4-phenylbutyl) piperidine] decreases brain injury after transient focal ischemia in rats. Stroke 27(11):2120–2123
Lockhart BP, Soulard P, Benicourt C, Privat A, Junien JL (1995) Distinct neuroprotective profiles for sigma ligands against N-methyl-D-aspartate (NMDA), and hypoxia-mediated neurotoxicity in neuronal culture toxicity studies. Brain Res 675(1–2):110–120. doi:0006-8993(95)00049-V [pii]
Li Z, Cui S, Zhang Z, Zhou R, Ge Y, Sokabe M, Chen L (2009) DHEA-neuroprotection and -neurotoxicity after transient cerebral ischemia in rats. J Cereb Blood Flow Metab 29(2):287–296. https://doi.org/10.1038/jcbfm.2008.118
Zhu L, Chi T, Zhao X, Yang L, Song S, Lu Q, Ji X, Liu P, Wang L, Zou L (2017) Xanthoceraside modulates neurogenesis to ameliorate cognitive impairment in APP/PS1 transgenic mice. J Physiol Sci. https://doi.org/10.1007/s12576-017-0561-9
Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP (2009) Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res 198(2):472–476. https://doi.org/10.1016/j.bbr.2008.11.036
Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M (2004) Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ 11(4):403–415. https://doi.org/10.1038/sj.cdd.4401365
Deng B, Gou X, Chen H, Li L, Zhong H, Xu H, Jiang F, Zhao Z, Wang Q, Xu L (2013) Targeted delivery of neurogenin-2 protein in the treatment for cerebral ischemia-reperfusion injury. Biomaterials 34(34):8786–8797. https://doi.org/10.1016/j.biomaterials.2013.07.076
Caruso RA, Rigoli L, Parisi A, Fedele F, Bonanno A, Paparo D, Querci A, Crisafulli C, Branca G, Venuti A (2013) Neutrophil-rich gastric carcinomas: light and electron microscopic study of 9 cases with particular reference to neutrophil apoptosis. Ultrastruct Pathol 37(3):164–170. https://doi.org/10.3109/01913123.2013.768746
Zhou L, Gao Q, Nie M, Gu JL, Hao W, Wang L, Cao JM (2016) Degeneration and energy shortage in the suprachiasmatic nucleus underlies the circadian rhythm disturbance in ApoE-/- mice: implications for Alzheimer’s disease. Sci Rep 6:36335. https://doi.org/10.1038/srep36335
Corpas R, Revilla S, Ursulet S, Castro-Freire M, Kaliman P, Petegnief V, Gimenez-Llort L, Sarkis C, Pallas M, Sanfeliu C (2016) SIRT1 overexpression in mouse hippocampus induces cognitive enhancement through proteostatic and neurotrophic mechanisms. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0087-9
Zhu L, Yang L, Zhao X, Liu D, Guo X, Liu P, Chi T, Ji X, Zou L (2017) Xanthoceraside modulates NR2B-containing NMDA receptors at synapses and rescues learning-memory deficits in APP/PS1 transgenic mice. Psychopharmacology. https://doi.org/10.1007/s00213-017-4775-6
Mitsuda T, Omi T, Tanimukai H, Sakagami Y, Tagami S, Okochi M, Kudo T, Takeda M (2011) Sigma-1Rs are upregulated via PERK/eIF2alpha/ATF4 pathway and execute protective function in ER stress. Biochem Biophys Res Commun 415(3):519–525. https://doi.org/10.1016/j.bbrc.2011.10.113
Wu CX, Liu R, Gao M, Zhao G, Wu S, Wu CF, Du GH (2013) Pinocembrin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress induced apoptosis. Neurosci Lett 546:57–62. https://doi.org/10.1016/j.neulet.2013.04.060
Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang J, Ai Q, Xing N, Sun X (2016) Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep 6:21730. https://doi.org/10.1038/srep21730
Xu Q, Ji XF, Chi TY, Liu P, Jin G, Gu SL, Zou LB (2015) Sigma 1 receptor activation regulates brain-derived neurotrophic factor through NR2A-CaMKIV-TORC1 pathway to rescue the impairment of learning and memory induced by brain ischaemia/reperfusion. Psychopharmacology 232(10):1779–1791. https://doi.org/10.1007/s00213-014-3809-6
Omi T, Tanimukai H, Kanayama D, Sakagami Y, Tagami S, Okochi M, Morihara T, Sato M, Yanagida K, Kitasyoji A, Hara H, Imaizumi K, Maurice T, Chevallier N, Marchal S, Takeda M, Kudo T (2014) Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis 5:e1332. https://doi.org/10.1038/cddis.2014.301
Zhang Z, Tong N, Gong Y, Qiu Q, Yin L, Lv X, Wu X (2011) Valproate protects the retina from endoplasmic reticulum stress-induced apoptosis after ischemia-reperfusion injury. Neurosci Lett 504(2):88–92. https://doi.org/10.1016/j.neulet.2011.09.003
Moskowitz MA, Kiwak KJ, Hekimian K, Levine L (1984) Synthesis of compounds with properties of leukotrienes C4 and D4 in gerbil brains after ischemia and reperfusion. Science 224(4651):886–889
Zhao X, Strong R, Zhang J, Sun G, Tsien JZ, Cui Z, Grotta JC, Aronowski J (2009) Neuronal PPARgamma deficiency increases susceptibility to brain damage after cerebral ischemia. J Neurosci 29(19):6186–6195. https://doi.org/10.1523/JNEUROSCI.5857-08.2009
Jing G, Wang JJ, Zhang SX (2012) ER stress and apoptosis: a new mechanism for retinal cell death. Exp Diabetes Res 2012:589589. https://doi.org/10.1155/2012/589589
Marwarha G, Dasari B, Ghribi O (2012) Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell Signal 24(2):484–492. https://doi.org/10.1016/j.cellsig.2011.09.029
Li H, Zhu X, Fang F, Jiang D, Tang L (2014) Down-regulation of GRP78 enhances apoptosis via CHOP pathway in retinal ischemia-reperfusion injury. Neurosci Lett 575:68–73. https://doi.org/10.1016/j.neulet.2014.05.042
Lin JH, Li H, Zhang Y, Ron D, Walter P (2009) Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 4(1):e4170. https://doi.org/10.1371/journal.pone.0004170
Darling NJ, Cook SJ (2014) The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim Biophys Acta 1843(10):2150–2163. https://doi.org/10.1016/j.bbamcr.2014.01.009
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131(3):596–610. https://doi.org/10.1016/j.cell.2007.08.036
Prell T, Lautenschlager J, Weidemann L, Ruhmer J, Witte OW, Grosskreutz J (2014) Endoplasmic reticulum stress is accompanied by activation of NF-kappaB in amyotrophic lateral sclerosis. J Neuroimmunol 270(1–2):29–36. https://doi.org/10.1016/j.jneuroim.2014.03.005
Schonthal AH (2013) Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol 85(5):653–666. https://doi.org/10.1016/j.bcp.2012.09.012
Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AF, Rothermel BA, Lavandero S (2012) Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol 44(1):16–20. https://doi.org/10.1016/j.biocel.2011.10.012
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276(17):13935–13940. https://doi.org/10.1074/jbc.M010677200
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277(37):34287–34294. https://doi.org/10.1074/jbc.M204973200
Wu H, Tang Q, Yang J, Ding J, Ye M, Dong W (2014) Atorvastatin ameliorates myocardial ischemia/reperfusion injury through attenuation of endoplasmic reticulum stress-induced apoptosis. Int J Clin Exp Med 7(12):4915–4923
Acknowledgements
This study was supported by the Research Fund for National Natural Science Foundation of China (No. 81503057), the Liaoning Province Doctor Startup Fund of the Science and Technology Department of Liaoning Province (No. 201601143), the Career Development Support Program for Young and Middle-aged Teachers of Shenyang Pharmaceutical University (No. ZQN2015030) and the Undergraduate Training Programs of Innovation and Entrepreneurship of Shenyang Pharmaceutical University (No. 201610163036).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhao, X., Zhu, L., Liu, D. et al. Sigma-1 receptor protects against endoplasmic reticulum stress-mediated apoptosis in mice with cerebral ischemia/reperfusion injury. Apoptosis 24, 157–167 (2019). https://doi.org/10.1007/s10495-018-1495-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1495-2