Abstract
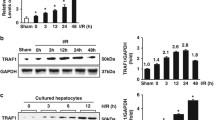
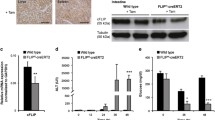
Hepatic ischemia-reperfusion injury remains a significant problem for liver surgery, including transplantation, and apoptosis has been implicated in this type of hepatic injury. Here we found that through the Fas/Fas ligand interaction apoptosis is involved in the late phase of hepatic ischemia-reperfusion injury. The appearance of apoptotic hepatocytes increases significantly after reperfusion, reaching a maximum 12 h after reperfusion. The transcription levels of Fas and Fas ligand are increased after reperfusion. Fas is expressed on hepatocytes, while Fas ligand is expressed on infiltrating immune cells. A close spatial and temporal association of Fas expression and apoptotic cells is demonstrated in the histological observation. These results suggest that infiltrating cells induce apoptosis of hepatocytes through the Fas/Fas ligand interaction, leading to hepatocyte injury. Furthermore, an injection of anti-Fas antibody or neutralizing anti-Fas ligand antibody results in a dramatic decrease in the occurrence of hepatocyte apoptosis and hepatic infiltration of macrophages and natural killer cells as well as liver injury. Our results suggest that blockage of the Fas/Fas ligand interaction is a promising strategy for suppression of hepatic ischemia-reperfusion injury.





Similar content being viewed by others
References
Bismuth H, Castaing D, Garden OJ (1989) Major hepatic resection under total vascular exclusion. Ann Surg 210:13–19. doi:10.1097/00000658-198907000-00002
Starzl T, Bell R, Beart R, Putnam R (1975) Hepatic trisegmentectomy and other liver resections. Surg Gynecol Obstet 141:429–437
Clavien PA, Harvey PRC, Strasberg SM (1992) Preservation and reperfusion injury allografts: overview and synthesis of current studies. Transplantation 53:957–978. doi:10.1097/00007890-199205000-00001
Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ (2000) Inflammatory mechanisms and therapeutic strategies for worm hepatic ischemia/reperfusion injury. Hepatology 32:169–173. doi:10.1053/jhep.2000.9323
Schwabe RF, Brenner DA (2006) Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 290:G583–G589. doi:10.1152/ajpgi.00422.2005
Jaeschke H, Smith CW (1997) Mechanisms of neutrophil induced parenchymal cell injury. J Leukoc Biol 61:647–653
Schlayer HJ, Laaff H, Peters T et al (1988) Involvement of tumor necrosis factor in endotoxin-triggered neutrophils adherence to sinusoidal endothelial cells of mouse liver and its modulation in acute phase. J Hepatol 7:239–249. doi:10.1016/S0168-8278(88)80488-4
Edelstein CL, Wieder ED, Yaqoob MM et al (1995) The role of cysteine proteases in hypoxia-induced rat renal proximal tubular injury. Proc Natl Acad Sci USA 92:7662–7666. doi:10.1073/pnas.92.17.7662
Nichols JC, Bronk SF, Gores GJ (1994) Inhibition of nonlysosomal calcium-dependent proteolysis by glycine during anoxic injury of rat hepatocytes. Gastroenterology 106:168–176
Kohli V, Gao W, Camargo CA, Clavien PA (1997) Calpain is a mediator of preservation: reperfusion injury in rat liver transplantation. Proc Natl Acad Sci USA 94:9354–9359. doi:10.1073/pnas.94.17.9354
Aguilar HI, Steers JL, Weisner RH, Krom RAF, Gores GJ (1997) Enhanced liver calpain protease activity is a risk factor for dysfunction of human liver allograft. Transplantation 63:612–614. doi:10.1097/00007890-199702270-00023
Vivek K, Markus S, John FM, Rex CB, Pierre AC (1999) Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation 67:1099–1105. doi:10.1097/00007890-199904270-00003
Li SQ, Liang LJ, Huang JH, Li Z (2003) Hepatocyte apoptosis induced by hepatic ischemia-reperfusion injury in cirrhotic rats. Hepatobiliary Pancreat Dis Int 2:102–105
Rudiger HA, Clavien PA (2002) Tumor necrosis factor alpha, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology 122:202–210. doi:10.1053/gast.2002.30304
Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL (1994) Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94:1621–1628. doi:10.1172/JCI117504
Itoh N, Yonehara S, Ishii A et al (1991) The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66:233–243. doi:10.1016/0092-8674(91)90614-5
Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75:1169–1178. doi:10.1016/0092-8674(93)90326-L
Kayagaki N, Kawasaki A, Ebata T et al (1995) Metalloproteinase-mediated release of human Fas ligand. J Exp Med 182:1777–1783. doi:10.1084/jem.182.6.1777
Serrao KL, Fortenberry JD, Owens ML, Harris FL, Brown LA (2001) Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am J Physiol Lung Cell Mol Physiol 280:L298–L305
Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ (1996) Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med 184:429–440. doi:10.1084/jem.184.2.429
Kiener PA, Davis PM, Rankin BM et al (1997) Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol 159:1594–1598
Nagata S (1997) Apoptosis by death factor. Cell 88:355–365. doi:10.1016/S0092-8674(00)81874-7
Kischkel FC, Hellbardt S, Behrmann I et al (1995) Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14:5579–5588
Medema JP, Scaffidi C, Kischkel FC et al (1997) FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J 16:2794–2804. doi:10.1093/emboj/16.10.2794
Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776. doi:10.1038/35037710
Susin SA, Lorenzo HK, Zamzami N et al (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441–446. doi:10.1038/17135
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489. doi:10.1016/S0092-8674(00)80434-1
Kagi D, Vignaux F, Ledermann B et al (1994) Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science 265:528–530. doi:10.1126/science.7518614
Lowin B, Hahne M, Mattmann C, Tschopp J (1994) Cytotoxic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370:650–652. doi:10.1038/370650a0
Rodriguez I, Matsuura K, Ody C, Nagata S, Vassalli P (1996) Systemic injection of a tripeptide inhibits the intracellular activation of CPP32-like proteases in vivo and fully protects mice against Fas-mediated fulminant liver destruction and death. J Exp Med 184:2067–2072. doi:10.1084/jem.184.5.2067
Eguchi S, Matsuzaki S, Fujioka H, Koji T, Higami Y, Kanematsu T (1998) The Fas/Fas-ligand system function in hepatocytes in the early stage of fulminant hepatic failure in rats. Hepatol Res 11:103–114. doi:10.1016/S1386-6346(98)00020-5
Tordjmann T, Soulie A, Guettier C et al (1998) Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver 18:391–397
Lin MT, Stohlman SA, Hinton DR (1997) Mouse hepatitis virus is cleared from the central nervous system of mice lacking perforin-mediated cytolysis. J Virol 71:383–391
Watanabe Y, Morita M, Akaike T (1996) Concanavalin A induces perforin-mediated but not Fas-mediated hepatic injury. Hepatology 24:702–710. doi:10.1002/hep.510240338
Kitamura Y, Hashimoto S, Mizuta N et al (2001) Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med 163:762–769
Albertine KH, Soulier MF, Wang Z et al (2002) Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol 161:1783–1796
Hashimoto S, Kobayashi A, Kooguchi K, Kitamura Y, Onodera H, Nakajima H (2000) Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med 161:237–243
Mizuta M, Nakajima H, Mizuta N et al (2008) Fas ligand released by activated monocytes causes apoptosis of lung epithelial cells in human acute lung injury model in vitro. Biol Pharm Bull 31:386–390. doi:10.1248/bpb.31.386
Hakuno N, Koji T, Yano T et al (1996) PKFas/APO-1/CD95 system as a mediator of granulosa cell apoptosis in ovarian follicle atresia. Endocrinology 137:1938–1948. doi:10.1210/en.137.5.1938
Koji T, Hishikawa Y, Ando H, Nakanishi Y, Kobayashi N (2001) Expression of Fas and Fas ligand in normal and ischemia-reperfusion testes: involvement of the Fas system in the induction of germ cell apoptosis in the damaged mouse testis. Biol Reprod 64:946–954. doi:10.1095/biolreprod64.3.946
Kayagaki N, Yamaguchi N, Nagao F et al (1997) Polymorphism of murine Fas ligand that affects the biological activity. Proc Natl Acad Sci USA 94:3914–3919. doi:10.1073/pnas.94.8.3914
Ejima K, Koji T, Tsuruta D, Nanri H, Kashimura M, Ikeda M (2000) Induction of apoptosis in placentas of pregnant mice exposed to lipopolysaccharides: possible involvement of Fas/Fas ligand system. Biol Reprod 62:178–185. doi:10.1095/biolreprod62.1.178
Seko Y, Kayagaki N, Seino K, Yagita H, Okumura K, Nagai R (2002) Role of Fas/FasL pathway in the activation of infiltrating cells in murine acute myocarditis caused by Coxsackievirus B3. J Am Coll Cardiol 39:1399–1403. doi:10.1016/S0735-1097(02)01776-X
Seino K, Iwabuchi K, Kayagaki N et al (1998) Chemotactic activity of soluble Fas ligand against phagocytes. J Immunol 161:4484–4488
Shibuya H, Ohkohchi N, Tsukamoto S, Satomi S (1997) Tumor necrosis factor-induced, superoxide-mediated neutrophils accumulation in cold ischemia/reperfused rat liver. Hepatology 26:113–120. doi:10.1002/hep.510260115
Rudiger HA, Clavien PA (2002) Tumor necrosis factor a, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology 122:202–210. doi:10.1053/gast.2002.30304
Stephen WC, Pauline DT, Daniel GR, Wendy ES, Steven LK (1991) In vivo biologic and immunohistochemical analysis of interleukin-1 α, β and tumor necrosis factor during experimental endotoxemia. Am J Pathol 138:395–402
Li SQ, Liang LJ, Huang JF, Li Z (2003) Hepatocyte apoptosis induced by hepatic ischemia-reperfusion injury in cirrhotic rats. Hepatobiliary Pancreat Dis Int 2:102–105
Li X, Zhang JF, Lu MQ, Yang Y, Xu C, Li H et al (2007) Alleviation of ischemia-reperfusion injury in rat liver transplantation by induction of small interference RNA targeting Fas. Langenbecks Arch Surg 392:345–351. doi:10.1007/s00423-006-0142-5
Mukhopadhyay A, Ni J, Zhai Y, Yu GL (1999) Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-κB, and c-Jun NH2-terminal kinase. J Biol Chem 274:15978–15981. doi:10.1074/jbc.274.23.15978
Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR (1998) DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-κB and AP-1. Mol Cell 1:543–551. doi:10.1016/S1097-2765(00)80054-4
Brown S, Savill J (1999) Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J Immunol 162:480–485
Park DR, Thomsen AR, Frevert CW et al (2003) Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 170:6209–6216
Tsutsui H, Matsui K, Okamura H, Nakanishi K (2000) Pathophysiological roles of interleukin-18 in inflammatory liver diseases. Immunol Rev 174:192–209. doi:10.1034/j.1600-0528.2002.017418.x
Morita M, Watanabe Y, Akaike T (1995) Protective effect of hepatocyte growth factor on interferon-γ induced cytotoxicity in mouse hepatocytes. Hepatology 21:1585–1593
Leithauser F, Dhein J, Mechtersheimer G et al (1993) Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily in normal and neoplastic cells. Lab Invest 69:415–429
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakajima, H., Mizuta, N., Fujiwara, I. et al. Blockade of the Fas/Fas ligand interaction suppresses hepatocyte apoptosis in ischemia-reperfusion rat liver. Apoptosis 13, 1013–1021 (2008). https://doi.org/10.1007/s10495-008-0234-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0234-5