Abstract
A wide range of bacterial species are able to induce calcium carbonate precipitation. Using our own laboratory-preserved strains, we have newly discovered that Ensifer sp. MY11e, Microbacterium sp. TMd9a1, Paeniglutamicibacter sp. MSa1a, Pseudomonas sp. GTc3, and Rheinheimera sp. ATWe6 can induce the formation of calcite crystals on an agar medium. Type strains of their closely related species (Ensifer adhaerens, Microbacterium testaceum, Paeniglutamicibacter kerguelensis, Pseudomonas protegens, and Rheinheimera texasensis) could also induce calcite formation. Although the initial pH value of the agar medium was 6.1, the pH of the agar media containing calcite, induced by cultivation of the 10 bacterial strains, increased to 8.0–8.4. The ammonification (oxidative deamination) of amino acids may been responsible for this increase in pH. The crystals formed both on and around the bacterial colonies. Furthermore, when these strains (excepting two Microbacterium strains) were cultivated on a cellulose acetate membrane filter (0.20 μm pore size) resting on the surface of the agar medium (i.e., in the membrane filter culture method), the crystals formed on the agar medium separate from the bacterial cells. These results indicate that the bacterial cells did not necessarily become nucleation sites for these crystals. We also investigated whether the studied strains could be applied to the biocementation of sand, and found that only two Ensifer strains were able to form large sand lumps.
Similar content being viewed by others
Introduction
Biomineralization is a process by which living organisms produce minerals. In one form of bacterial biomineralization, bacteria induce calcium carbonate precipitation. The most common bacterial calcium carbonate polymorphs are calcite and vaterite (Dhami et al. 2013a). Bacterial calcium carbonate precipitation occurs extracellularly as a result of the bacteria’s metabolic activities and surrounding environment. This biologically induced mineralization differs from biologically controlled mineralization, such as the formation of magnetic minerals by magnetotactic bacteria (Lowenstam 1981; Lowenstam and Weiner 1989; Bazylinski and Frankel 2003).
The possible mechanisms of bacterial calcium carbonate precipitation have been elucidated (De Muynck et al. 2010; Dhami et al. 2013a; Ganendra et al. 2014; Anbu et al. 2016; Zhu and Dittrich 2016). Bacterial calcium carbonate precipitation can occur as a by-product of common bacterial metabolic activities, including photosynthesis, ureolysis, denitrification, ammonification, sulfate reduction, and formate oxidation. These metabolic activities cause an increase in pH or dissolved inorganic carbon, rendering the microenvironment around the bacterial cells suitable for calcium carbonate precipitation, and enabling the bacterial cell surfaces to become nucleation sites for carbonate precipitation. Cell walls with negatively charged functional groups, such as carboxyl and phosphate groups, are then able to adsorb calcium ions, and carbonates will then precipitate on the cell surface when carbonate species are available. In addition, extracellular polymeric substances may play an important role in the morphology and mineralogy of calcium carbonate precipitation.
Bacterial calcium carbonate precipitation has potential biotechnological applications in the removal of heavy metals and radionuclides from contaminated sites, bioconsolidation of soil and sand, biocementation, and CO2 sequestration (Dhami et al. 2013a; De Muynck et al. 2010; Anbu et al. 2016; Zhu and Dittrich 2016). A majority of the studies on such biotechnological applications (excepting CO2 sequestration) were based on ureolysis (Zhu and Dittrich 2016).
Various bacteria capable of inducing calcium carbonate precipitation have been reported. In particular, cyanobacteria were reported to induce calcium carbonate precipitation through photosynthesis (Jansson and Northen 2010). Numerous ureolytic bacteria have been investigated in this respect, including members of the genera Arthrobacter, Bacillus, Brevibacterium, Brevundimonas, Enterobacter, Exiguobacterium, Lysinibacillus, Pseudomonas and Sporosarcina (Park et al. 2010; Dhami et al. 2013b; Ghashghaei and Emtiazi 2013; Heidari Nonakaran et al. 2015; Wei et al. 2015; Bansal et al. 2016; Zhu et al. 2017). Halomonas halodenitrificans has been identified as a denitrifying bacterium that could induce calcium carbonate precipitation under anaerobic conditions (Martin et al. 2013), and the members of the genera Bacillus, Cupriavidus, Pseudomonas, Pantoea, Myxococcus, and Streptomyces induced calcium carbonate precipitation by ammonification (oxidative deamination) of amino acids (González-Muñoz et al. 2010; Daskalakis et al. 2013; Maciejewska et al. 2017). Desulfovibrio alaskensis has been identified as a sulfate-reducing bacterium that can promote carbonate precipitation (Bosak and Newman 2005), and formate oxidation-driven calcium carbonate precipitation by Methylocystis parvus has also been reported (Ganendra et al. 2014). The isolation sources of the abovementioned bacteria were various [e.g., soil samples (Dhami et al. 2013b; Ghashghaei and Emtiazi 2013; Heidari Nonakaran et al. 2015), concrete structures (Park et al. 2010), stone (Daskalakis et al. 2013), cave moonmilk (Maciejewska et al. 2017), marine sediment (Wei et al. 2015; Zhu et al. 2017), and sea water (Bansal et al. 2016)].
We had previously isolated various bacterial strains from numerous environmental samples in other studies, and had preserved these in our laboratory; however, we did not determine their ability to induce calcium carbonate precipitation. In this study, we considered the possibility that unreported bacteria capable of inducing calcium carbonate precipitation may exist among our laboratory-preserved strains, belonging to various taxa, and sought to identify and investigate these strains.
Materials and methods
Bacterial strains
Ensifer sp. MY11e (= NBRC 109443) was isolated from a soil sample collected from a paddy field in Kamakura, Kanagawa Prefecture, Japan (GPS coordinates not recorded; sampling date: September 11, 2009). Microbacterium sp. TMd9a1 was isolated from a freshwater sample collected from the Tamagawa River in Kanagawa Prefecture, Japan (GPS coordinates: 35°26′07.5″N 139°18′47.4″E; sampling date: May 5, 2013). Paeniglutamicibacter sp. MSa1a was isolated from a soil sample collected from the Nanasawa Forest Park in Kanagawa Prefecture, Japan (GPS coordinates: 35°26′41.3″N 139°18′10.2″E; sampling date: March 13, 2013). Pseudomonas sp. GTc3 was isolated from a soil sample collected from the Tanigawadake Tenjindaira area in Gunma Prefecture, Japan (GPS coordinates: 36°49′05.9″N 138°57′09.5″E; sampling date: May 13, 2013). Rheinheimera sp. ATWe6 was isolated from a freshwater sample collected from the Koayugawa River in Kanagawa Prefecture, Japan (GPS coordinates: 35°27′38.5″N 139°21′08.0″E; sampling date: March 17, 2013). These strains were preserved as glycerol suspensions (20 %, v/v) at − 80 °C, and routinely cultured aerobically on R2A agar or in liquid R2A medium (Reasoner and Geldreich 1985) for 2–4 days at 28 °C.
Ensifer adhaerens JCM 21105T, Microbacterium testaceum JCM 1353T, and Paeniglutamicibacter kerguelensis JCM 12165T were obtained from the Japan Collection of Microorganisms. Pseudomonas protegens DSM 19095T and Rheinheimera texasensis DSM 17496T were obtained from the Leibniz Institute DSMZ–German Collection of Microorganisms and Cell Cultures.
Determination of 16S rRNA gene sequence
Genomic DNA was extracted using an UltraClean Microbial DNA Isolation Kit (Mo Bio Laboratories Inc., Carlsbad, CA, USA). PCR amplification of the 16S rRNA gene and sequencing were performed as previously described (Hatayama et al. 2005). A similarity-based search was performed using the EzBioCloud server (Yoon et al. 2017).
Nucleotide sequence accession numbers
The GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequences of Ensifer sp. MY11e, Microbacterium sp. TMd9a1, Paeniglutamicibacter sp. MSa1a, Pseudomonas sp. GTc3 and Rheinheimera sp. ATWe6 are AB678697, LC406749, LC406750, LC406751 and LC406752, respectively.
Calcium carbonate precipitation
Each bacterial strain was inoculated on MRC agar [0.5 g of yeast extract, 0.5 g of proteose peptone (Difco No. 3), 0.5 g of casamino acids, 0.5 g of glucose, 0.5 g of soluble starch, 0.3 g of sodium pyruvate, 2.5 g of calcium chloride dehydrate, 15 g of agar, and 1 L of deionized water] (pH was not adjusted; measured value: pH 6.1), and aerobically cultivated at 28 °C for 7 days. The calcium carbonate crystals that formed on the MRC agar were observed under a phase-contrast light microscope (BH-2, Olympus, Tokyo, Japan) at 100 × magnification. After the cultivation period, the pH value of the MRC agar was measured using a flat ISFET pH electrode (0040-10D, Horiba Ltd., Kyoto, Japan).
Harvest of calcium carbonate crystals from MRC agar plates
The calcium carbonate crystals were harvested from the MRC agar plates after the cultivation period. First, the bacteriolytic procedure, using lysozyme and sodium dodecyl sulfate (SDS), was performed. A 10 mL portion of lysozyme solution [100 mg/L of lysozyme with 50 mM Tris–HCl (pH 8.0)] was added to the plate, and grown bacterial cells were suspended in it using a spreader. The plate was then incubated at 37 °C for 1 h. A 1 mL portion of 10% (w/v) SDS solution was then mixed into the suspension, and the plate was again incubated at 37 °C for 1 h. After the bacteriolytic procedure, the suspension and crushed MRC agar were transferred to a conical beaker. A 60 mL portion of deionized water (per MRC agar plate) was added to the conical beaker, which was then autoclaved at 121 °C for 20 min. The supernatant containing melted MRC agar was removed by decantation, and the precipitates were washed several times with deionized water, and freeze-dried.
The membrane filter culture method
Cellulose acetate membrane filters (0.20 μm pore size, 125 μm thickness) were sterilized by autoclaving. Then, for each strain, a sterile cellulose acetate membrane filter was floated on melted MRC agar by autoclaving, and the agar was solidified at room temperature; after which, a bacterial strain was inoculated on the cellulose acetate membrane filter of the agar, and aerobically cultivated at 28 °C for 7 days. After the cultivation period, the cellulose acetate membrane filter with grown bacterial cells was removed from the agar, and the calcium carbonate crystals that had formed in the agar were observed under a phase-contrast light microscope at 100 × magnification. The crystals were harvested from the melted agar by heating at 95 °C for 20 min.
Observation of calcite crystals
The morphology of the calcium carbonate crystals was observed using a low-vacuum scanning electron microscope (LV-SEM) (Hitachi Tabletop Microscope TM-1000, Hitachi High-Technologies Corp., Tokyo, Japan).
X-ray diffraction (XRD) analysis
XRD patterns were collected at room temperature on a powder X-ray diffractometer (SmartLab-3 kW, Rigaku Corp., Tokyo, Japan) with Cu Kα radiation (40 kV and 30 mA) in the 2θ range of 5°–50°, at a step of 0.02°, with a scanning rate of 20°min−1. The XRD patterns were analyzed by PDXL 2 software (version 2.3.1.0, Rigaku Corp.) together with the ICDD PDF-2 database (released 2014).
Biocementation of sand
Conical tubes (50 mL) containing 15 g of silica sand (grain size: 80–300 μm) (JFE Mineral Co. Ltd., Tokyo, Japan) were autoclaved at 121 °C for 20 min. A 3 mL portion of MRC liquid medium (as for the MRC agar, but without agar) containing a bacterial strain (optical density at 600 nm: approximately 0.1) was perfused into the silica sand in the conical tube, and the tube was then incubated at 28 °C for 11 days. After the incubation period, the silica sand samples were dried at 60 °C for 2 days, and the lumps of biocemented sand were separated using a testing sieve (aperture: 1.0 mm).
Scanning electron microscope (SEM) and energy-dispersive X-ray spectroscopy (EDS) analyses
The SEM and EDS analyses were conducted by Tokai Electron Microscopy, Inc. (Aichi, Japan). The biocemented sand sample was coated with a thin layer of carbon using a vacuum evaporator (JEE-4X, JEOL Ltd., Tokyo, Japan), and then observed by SEM (JSM-7500F, JEOL). The elemental components of the sample were analyzed by EDS (JED-2300F, JEOL).
Results
Calcium carbonate precipitations induced by laboratory-preserved strains
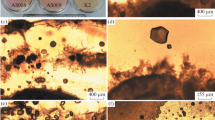
We had previously isolated, from various environmental samples, approximately 100 bacterial strains, belonging to the phyla Actinobacteria, Bacteroidetes, Firmicutes, or Proteobacteria (Hatayama et al. 2013, 2016; Hatayama 2014; Hatayama and Kuno 2015a, b), and these strains were preserved in our laboratory. We considered the possibility that some were capable of inducing calcium carbonate precipitation, and thus screened for strains that can induce such precipitation on MRC agar. The MRC agar was developed by modifying R2A agar for bacterial calcium carbonate precipitation, because the preserved strains grew well on R2A agar. A total of five target strains were obtained from the screening. From a similarity-based search of 16S rRNA gene sequences, the five strains were identified as Ensifer sp. MY11e (closely related species and 16S rRNA gene sequence similarity: E. adhaerens Casida AT, 99.7%), Microbacterium sp. TMd9a1 (M. testaceum DSM 20166T, 99.8%), Paeniglutamicibacter sp. MSa1a (Pa. kerguelensis KGN15T, 99.0%), Pseudomonas sp. GTc3 (Ps. protegens CHA0T, 100%), and Rheinheimera sp. ATWe6 (R. texasensis A62-14BT, 98.7%). The calcium carbonate crystal formations induced by the five strains on MRC agar plates were observed not only in terms of the colonies but also the surrounding region (Fig. 1a–c, g, h). The respective pH values of the MRC agar media for Ensifer sp. MY11e, Microbacterium sp. TMd9a1, Paeniglutamicibacter sp. MSa1a, Pseudomonas sp. GTc3, and Rheinheimera sp. ATWe6, after the cultivation period, were 8.2, 8.1, 8.0, 8.4, and 8.4. Ensifer sp. MY11e, Microbacterium sp. TMd9a1, Paeniglutamicibacter sp. MSa1a, and Rheinheimera sp. ATWe6 formed polyhedral crystals (resembling truncated octahedrons, octahedrons, oval truncated cuboctahedrons, and truncated cuboctahedrons, respectively) covered with small rhombohedra (crystal size: 10–50 μm, 10–100 μm, 30–400 μm, and 10–70 μm, respectively) (Fig. 1d–f, j). Morphologies characterized by clusters of needles, fuzzy fibers, and clumps of dust were observed on the surface of crystals induced by Pseudomonas sp. GTc3 (Fig. 1i, k, l) (crystal size: 40–400 μm). XRD analysis confirmed that all the calcium carbonate crystals induced by the five strains were calcite (Fig. 2).
Microscopic images of calcium carbonate crystals. a–c, g, h phase-contrast light microscope images of calcium carbonate crystals formed on MRC agar media cultivated with Ensifer sp. MY11e (a), Microbacterium sp. TMd9a1 (b), Paeniglutamicibacter sp. MSa1a (c), Rheinheimera sp. ATWe6 (g), and Pseudomonas sp. GTc3 (h); (C) and (M) indicate colony and non-colony regions on the MRC agar, respectively. d–f, i–l LV-SEM images of calcium carbonate crystals induced by Ensifer sp. MY11e (d), Microbacterium sp. TMd9a1 (e), Paeniglutamicibacter sp. MSa1a (f), Rheinheimera sp. ATWe6 (j), and Pseudomonas sp. GTc3 (i, k, l). The morphologies characterized by clusters of needles (k), fuzzy fibers (l), and clumps of dust (i) were observed on the surface of calcium carbonate crystals induced by Pseudomonas sp. GTc3. Scale bars in a–c, g, h 200 μm. Other scale bars 30 μm
Calcium carbonate precipitations induced by type strains
We next investigated whether the calcium carbonate precipitations induced by the five laboratory strains were peculiar to these strains. Therefore, calcium carbonate precipitations induced by type strains of species closely related to the five target strains were examined. Based on a similarity-based search of 16S rRNA gene sequences, E. adhaerens JCM 21105T, M. testaceum JCM 1353T, Pa. kerguelensis JCM 12165T, Ps. protegens DSM 19095T, and R. texasensis DSM 17496T were used as the type strains. After cultivation on MRC agar, these type strains formed calcium carbonate crystals on and around their colonies (Supplementary Fig. S1a–c, g, h). The respective pH values of the MRC agar media for E. adhaerens JCM 21105T, M. testaceum JCM 1353T, Pa. kerguelensis JCM 12165T, Ps. protegens DSM 19095T, and R. texasensis DSM 17496T, after the cultivation period, were 8.2, 8.3, 8.2, 8.3, and 8.3. The respective morphologies of the calcium carbonate crystals induced by E. adhaerens JCM 21105T (crystal size: 10–50 μm), M. testaceum JCM 1353T (10–70 μm), Ps. protegens DSM 19095T (40–400 μm), and R. texasensis DSM 17496T (10–100 μm) resembled those of Ensifer sp. MY11e, Microbacterium sp. TMd9a1, Pseudomonas sp. GTc3, and Rheinheimera sp. ATWe6 (Fig. 1d, e, i–l; Supplementary Fig. S1d, e, i–l). Pa. kerguelensis JCM 12165T induced calcium carbonate crystals of barrel shape with a rough surface (crystal size: 30–400 μm) (Supplementary Fig. S1f). All the calcium carbonate crystals induced by the five type strains were calcite (Supplementary Fig. S2).
Calcium carbonate precipitation by the membrane filter culture method
When any one of the five laboratory-preserved strains, or its closely related type strain, was cultivated on MRC agar, calcite crystals formed beyond the periphery of its colonies (Fig. 1a–c, g, h; Supplementary Fig. S1a–c, g, h). From this it was inferred that the bacterial cells themselves did not function as nucleation sites for these crystals. To confirm this, we tested whether calcite crystals would form in a portion of the MRC agar separate from the bacterial cells. Specifically, each of the strains was cultivated on a cellulose acetate membrane filter resting on the surface of MRC agar (Fig. 3a) (i.e., the membrane filter culture method). The bacterial cells were separated from the remainder of the MRC agar by the membrane filter (0.20 μm pore size), which could block the passage of bacteria.
A photograph of MRC agar in the membrane filter culture method (a), arrow indicates the cellulose acetate membrane filter (cultivated strain: Pseudomonas sp. GTc3); and LV-SEM images of calcium carbonate crystals formed using this method, with Ensifer sp. MY11e (b), E. adhaerens JCM 21105T (c), Paeniglutamicibacter sp. MSa1a (d), Pa. kerguelensis JCM 12165T (e), Pseudomonas sp. GTc3 (f), Ps. protegens DSM 19095T (g), Rheinheimera sp. ATWe6 (h), and R. texasensis DSM 17496T (i). Scale bar 50 μm
All five laboratory-preserved strains, and their closely related type strains, grew on the membrane filters, but none could pass through the cellulose acetate membrane filter. This was confirmed by additional incubation (at 28 °C for 7 days) of the MRC agar after removal of the filter. In the membrane filter culture method, polyhedral crystals covered with small rhombohedra were observed in the MRC agar after cultivation of Ensifer sp. MY11e (polyhedral morphology: truncated octahedron; crystal size: 20–100 μm, Fig. 3b), Paeniglutamicibacter sp. MSa1a (barrel shaped truncated cuboctahedron, 100–150 μm, Fig. 3d), Pseudomonas sp. GTc3 (truncated octahedron, 20–120 μm, Fig. 3f), Rheinheimera sp. ATWe6 (oval truncated cuboctahedron, 30–100 μm, Fig. 3h), E. adhaerens JCM 21105T (truncated octahedron, 50–100 μm, Fig. 3c), Ps. protegens DSM 19095T (truncated octahedron, 20–100 μm, Fig. 3g), and R. texasensis DSM 17496T (oval truncated cuboctahedron, 30–100 μm, Fig. 3i). Barrel shaped crystals with a rough surface were observed in the MRC agar after cultivation of Pa. kerguelensis JCM 12165T in this method (crystal size: 30–120 μm) (Fig. 3e). These results indicate that the bacterial cells did not necessarily become nucleation sites for these crystals, supporting the abovementioned inference. Calcite crystals did not form in the MRC agar when Microbacterium sp. TMd9a1 or M. testaceum JCM 1353T was cultivated using the membrane filter culture method.
Interestingly, the morphologies characterized by clusters of needles, fuzzy fibers, and clumps of dust were not observed on the surface of crystals formed in the MRC agar when Pseudomonas sp. GTc3 or Ps. protegens DSM 19095T was cultivated using the membrane filter culture method (Fig. 3f, g). In this method, the morphology of the crystals induced by the two Pseudomonas strains resembled that of the two Ensifer species (Fig. 3b, c, f, g).
Biocementation of sand
We also investigated whether the calcium carbonate precipitations induced by the studied strains could be applied to biocementation, a biotechnological application. Respective MRC liquid media, each containing one of the strains, were perfused into silica sand, which was then incubated at 28 °C for 11 days. After the incubation period, we examined the lumps of sand formed by biocementation. Sterilized deionized water and MRC liquid medium without bacteria were used as negative controls; and in each case no sand lumps were formed. Sand lumps were formed by using E. adhaerens JCM 21105T (weight of lumps: 2.6 g) (Fig. 4a), Ensifer sp. MY11e (2.4 g), Pa. kerguelensis JCM 12165T (0.7 g), Paeniglutamicibacter sp. MSa1a (0.5 g), or Microbacterium sp. TMd9a1 (0.3 g). No other strains induced sand lumps. Angular structures with morphologies differing from that of silica sand were observed on the lumps of sand induced by the Ensifer strains. These structures were not observed in the original silica sand. Therefore, it was thought that their formation was induced by the Ensifer strains. In one of these lumps, a plate binding two grains of sand was observed, with angular structures on its surface (Fig. 4b). In EDS analysis, calcium and sulfur were detected on the plate (Fig. 4c, Supplementary Fig. S3). Contrary to expectations, the binder of the silica sand particles might not be calcium carbonate, because sulfur was detected. The number of these angular structures was small in comparison with that of silica sand; thus, the XRD pattern of the lump of sand induced by E. adhaerens JCM 21105T was similar to that of the original silica sand (i.e., no XRD pattern was detected for these angular structures). We could not identify the mineral composition of these angular structures.
Discussion
Boquet et al. (1973) inferred that crystal formation of calcium carbonate is a function of the medium, and that under suitable conditions most bacteria can form crystals. It has been reported that various bacteria can induce calcium carbonate precipitation under laboratory conditions. However, it is thought that many bacteria capable of inducing calcium carbonate precipitation have yet to be identified and investigated. We considered the possibility that some such bacteria existed in our laboratory-preserved strains, and therefore screened the preserved strains, identifying five capable of inducing such precipitation. It had hitherto not been known whether their closely related species (E. adhaerens, M. testaceum, Pa. kerguelensis, Ps. protegens, and R. texasensis) were also able to induce calcium carbonate precipitation; and the present study confirmed that type strains of these species were indeed capable of such induction. Consequently, five new taxa of bacteria capable of inducing calcium carbonate precipitation were discovered.
All five laboratory-preserved strains, as well as their five closely related type strains, induced calcite formation, one of the crystal polymorphisms of calcium carbonate, on the MRC agar. Although the initial pH value of the MRC agar medium was 6.1, the pH of the MRC agar media containing calcite, induced by cultivation of the 10 bacterial strains, increased to 8.0–8.4. The increase in environmental pH results in the generation of carbonate ions which precipitate with calcium ions (Ca2+) (Anbu et al. 2016). Therefore, it was thought that the observed increase in the MRC agar medium pH level contributed to the formation of calcite. From their physiological characteristics (Komagata and Iizuka 1964; Casida 1982; Gupta et al. 2004; Merchant et al. 2007; Ramette et al. 2011) and their aerobic growth on MRC agar containing no urea, the mechanism of the pH increase would not relate to photosynthesis, ureolysis, denitrification, sulfate reduction, or formate oxidation. The probable mechanism behind the pH increase in the 10 studied strains was ammonium release by ammonification (oxidative deamination) of amino acids (Daskalakis et al. 2013), because the MRC agar contained amino acid-rich components such as yeast extract, proteose peptone (Difco No. 3), and casamino acids.
All 10 studied strains induced calcite crystal formation beyond the periphery of their MRC agar colonies. In addition, calcite crystals formed in MRC agar regions separate from the bacterial cells when Ensifer sp. MY11e, E. adhaerens JCM 21105T, Paeniglutamicibacter sp. MSa1a, Pa. kerguelensis JCM 12165T, Pseudomonas sp. GTc3, Ps. protegens DSM 19095T, Rheinheimera sp. ATWe6, or R. texasensis DSM 17496T was cultivated using the membrane filter culture method. These results indicate that the bacterial cells did not necessarily become nucleation sites for these crystals. On the other hand, calcite crystals did not form apart from the bacterial cells in MRC agar when Microbacterium sp. TMd9a1 or M. testaceum JCM 1353T was cultivated using the membrane filter culture method. The pH values of the MRC agar after their cultivation using the membrane filter method were 8.4, similar to those of the MRC agar containing calcite crystals formed by cultivation of these two strains. This result might indicate that the secretions of these strains, which could become crystalline nuclei, could not pass through the cellulose acetate membrane filter.
Aloisi et al. (2006) reported that bacterial nanoglobules (60–200 nm in diameter) emerging from bacterial cell surfaces could act as calcium carbonate nucleation sites once released into the culture medium; and it has recently been learned that most bacteria release nano-sized membrane vesicles into the extracellular environment (Kim et al. 2015). Possibly, then, such vesicles may have been secreted from the strains in this study, and become nucleation sites of calcite crystals. Specifically, the membrane vesicles from two of the Microbacterium strains might not have been able to pass through the cellulose acetate membrane filter, while those of the other eight strains might be able to do so.
In the case of Ensifer sp. MY11e, E. adhaerens JCM 21105T, Paeniglutamicibacter sp. MSa1a, Pa. kerguelensis JCM 12165T, Rheinheimera sp. ATWe6, and R. texasensis DSM 17496T, the morphologies of the calcite crystals formed on the MRC agar resembled those of the crystals formed on the MRC agar using the membrane filter method (Figs. 1, 3, Supplementary Fig. S1), suggesting that the morphologies were little affected by the presence of the membrane filter on the MRC agar. In the case of Pseudomonas sp. GTc3 and Ps. protegens DSM 19095T, however, the morphologies of the calcite crystals formed on the MRC agar markedly differed from those in the membrane filter method (Figs. 1i, k, l, 3f, g; Supplementary Fig. S1i, k, l). The morphologies characterized by clusters of needles, fuzzy fibers, and clumps of dust were observed only on the surface of calcite crystals formed on the MRC agar, indicating that unidentified substances from cells of these two Pseudomonas strains, incapable of passing through the membrane filter, were required for the formation of these characteristic morphologies. Greater control of calcite crystal morphology may thus be possible if these substances could be identified and exploited.
Large sand lumps were induced by two strains of the genus Ensifer in the biocementation investigation. The angular structures induced by E. adhaerens JCM 21105T were observed on the grains of sand in one of the lumps, and it was thought that these structures bound the grains of sand. They might not be composed of calcium carbonate, however, because EDS analysis revealed that not only calcium but also sulfur was detected in the structures. Unfortunately, the mineral composition of these angular structures could not be determined in this study. The reason why the Ensifer strains formed these sulfur-containing angular structures was unclear.
Although the binder of the sand grains was not identified, these two Ensifer strains may be useful for biotechnological applications, such as the restoration of construction material (Zhu and Dittrich 2016). The other eight studied strains, on the other hand, either did not form lumps of sand at all, or formed only small lumps, in the biocementation test. This result suggests that calcium carbonate precipitations capable of being sand-grain binder may be hardly induced on the sand surface by these strains. These strains may only be able to induce significant calcium carbonate precipitation under suitable conditions, such as cultivation on MRC agar; however, the results suggest that the ability to induce calcite precipitation on MRC agar was not directly correlated with biocementation capability.
Finally, the environmental sources of the 10 studied strains were samples of soils, freshwater, sea water, Chinese paddies, and tobacco roots (Komagata and Iizuka 1964; Casida 1982; Gupta et al. 2004; Merchant et al. 2007; Ramette et al. 2011). Thus, it appears that bacteria capable of inducing calcium carbonate precipitation on MRC agar exist in a variety of environments. Moreover, many bacteria with this capability may well be present in other laboratory-preserved collections, as in this study.
References
Aloisi G, Gloter A, Krüger M, Wallmann K, Guyot F, Zuddas P (2006) Nucleation of calcium carbonate on bacterial nanoglobules. Geology 34:1017–1020
Anbu P, Kang C-H, Shin Y-J, So J-S (2016) Formations of calcium carbonate minerals by bacteria and its multiple applications. Springerplus 5:250
Bansal R, Dhami NK, Mukherjee A, Reddy MS (2016) Biocalcification by halophilic bacteria for remediation of concrete structures in marine environment. J Ind Microbiol Biotechnol 43:1497–1505
Bazylinski DA, Frankel RB (2003) Biologically controlled mineralization in prokaryotes. Rev Mineral Geochem 54:217–247
Boquet E, Boronat A, Ramos-Cormenzana A (1973) Production of calcite (calcium carbonate) crystals by soil bacteria is a general phenomenon. Nature 246:527–529
Bosak T, Newman DK (2005) Microbial kinetic controls on calcite morphology in supersaturated solutions. J Sediment Res 75:190–199
Casida LE (1982) Ensifer adhaerens gen. nov., sp. nov.: a bacterial predator of bacteria in soil. Int J Syst Bacteriol 32:339–345
Daskalakis MI, Magoulas A, Kotoulas G, Catsikis I, Bakolas A, Karageorgis AP, Mavridou A, Doulia D, Rigas F (2013) Pseudomonas, Pantoea and Cupriavidus isolates induce calcium carbonate precipitation for biorestoration of ornamental stone. J Appl Microbiol 115:409–423
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136
Dhami NK, Reddy MS, Mukherjee A (2013a) Biomineralization of calcium carbonates and their engineered applications: a review. Front Microbiol 4:314
Dhami NK, Reddy MS, Mukherjee A (2013b) Biomineralization of calcium carbonate polymorphs by the bacterial strains isolated from calcareous sites. J Microbiol Biotechnol 23:707–714
Ganendra G, De Muynck W, Ho A, Arvaniti EC, Hosseinkhani B, Ramos JA, Rahier H, Boon N (2014) Formate oxidation-driven calcium carbonate precipitation by Methylocystis parvus OBBP. Appl Environ Microbiol 80:4659–4667
Ghashghaei S, Emtiazi G (2013) Production of calcite nanocrystal by a urease-positive strain of Enterobacter ludwigii and study of its structure by SEM. Curr Microbiol 67:406–413
González-Muñoz MT, Rodriguez-Navarro C, Martínez-Ruiz F, Arias JM, Merroun ML, Rodriguez-Gallego M (2010) Bacterial biomineralization: new insights from Myxococcus-induced mineral precipitation. Geol Soc Spec Publ 336:31–50
Gupta P, Reddy GS, Delille D, Shivaji S (2004) Arthrobacter gangotriensis sp. nov. and Arthrobacter kerguelensis sp. nov. from Antarctica. Int J Syst Evol Microbiol 54:2375–2378
Hatayama K (2014) Comamonas humi sp. nov., isolated from soil. Int J Syst Evol Microbiol 64:3976–3982
Hatayama K, Kuno T (2015a) Spirosoma fluviale sp. nov., isolated from river water. Int J Syst Evol Microbiol 65:3447–3450
Hatayama K, Kuno T (2015b) Croceifilum oryzae gen. nov., sp. nov., isolated from rice paddy soil. Int J Syst Evol Microbiol 65:4061–4065
Hatayama K, Kawai S, Shoun H, Ueda Y, Nakamura A (2005) Pseudomonas azotifigens sp. nov., a novel nitrogen-fixing bacterium isolated from a compost pile. Int J Syst Evol Microbiol 55:1539–1544
Hatayama K, Esaki K, Ide T (2013) Cellulomonas soli sp. nov. and Cellulomonas oligotrophica sp. nov., isolated from soil. Int J Syst Evol Microbiol 63:60–65
Hatayama K, Ushida A, Kuno T (2016) Flavobacterium aquicola sp. nov., isolated from river water. Int J Syst Evol Microbiol 66:2789–2796
Heidari Nonakaran S, Pazhouhandeh M, Keyvani A, Abdollahipour FZ, Shirzad A (2015) Isolation and identification of Pseudomonas azotoformans for induced calcite precipitation. World J Microbiol Biotechnol 31:1993–2001
Jansson C, Northen T (2010) Calcifying cyanobacteria—the potential of biomineralization for carbon capture and storage. Curr Opin Biotechnol 21:365–371
Kim JH, Lee J, Park J, Gho YS (2015) Gram-negative and gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol 40:97–104
Komagata K, Iizuka H (1964) New species of Brevibacterium isolated from rice (Studies on the microorganisms of cereal grains. Part VII). Nippon Nogeikagaku Kaishi 38:496–502 (in Japanese)
Lowenstam HA (1981) Minerals formed by organisms. Science 211:1126–1131
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press, Oxford
Maciejewska M, Adam D, Naômé A, Martinet L, Tenconi E, Całusińska M, Delfosse P, Hanikenne M, Baurain D, Compère P, Carnol M, Barton HA, Rigali S (2017) Assessment of the potential role of Streptomyces in cave moonmilk formation. Front Microbiol 8:1181
Martin D, Dodds K, Butler IB, Ngwenya BT (2013) Carbonate precipitation under pressure for bioengineering in the anaerobic subsurface via denitrification. Environ Sci Technol 47:8692–8699
Merchant MM, Welsh AK, McLean RJ (2007) Rheinheimera texasensis sp. nov., a halointolerant freshwater oligotroph. Int J Syst Evol Microbiol 57:2376–2380
Park S-J, Park Y-M, Chun W-Y, Kim W-J, Ghim S-Y (2010) Calcite-forming bacteria for compressive strength improvement in mortar. J Microbiol Biotechnol 20:782–788
Ramette A, Frapolli M, Fischer-Le Saux M, Gruffaz C, Meyer J-M, Défago G, Sutra L, Moënne-Loccoz Y (2011) Pseudomonas protegens sp. nov., widespread plant-protecting bacteria producing the biocontrol compounds 2,4-diacetylphloroglucinol and pyoluteorin. Syst Appl Microbiol 34:180–188
Reasoner DJ, Geldreich EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Wei S, Cui H, Jiang Z, Liu H, He H, Fang N (2015) Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz J Microbiol 46:455–464
Yoon S-H, Ha S-M, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA and whole genome assemblies. Int J Syst Evol Microbiol 67:1613–1617
Zhu T, Dittrich M (2016) Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: a review. Front Bioeng Biotechnol 4:4
Zhu Y, Ma N, Jin W, Wu S, Sun C (2017) Genomic and transcriptomic insights into calcium carbonate biomineralization by marine actinobacterium Brevibacterium linens BS258. Front Microbiol 8:602
Author information
Authors and Affiliations
Contributions
KH conceived of the study, carried out the experiments and wrote the manuscript. KS contributed to the experiments, discussion and preparation of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hatayama, K., Saito, K. Calcite formation induced by Ensifer adhaerens, Microbacterium testaceum, Paeniglutamicibacter kerguelensis, Pseudomonas protegens and Rheinheimera texasensis. Antonie van Leeuwenhoek 112, 711–721 (2019). https://doi.org/10.1007/s10482-018-1204-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1204-8