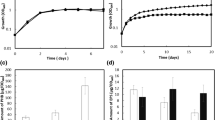
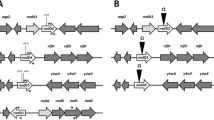
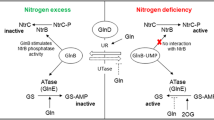
Abstract
Bradyrhizobium diazoefficiens, a nitrogen-fixing endosymbiont of soybeans, is a model strain for studying rhizobial denitrification. This bacterium can also use nitrate as the sole nitrogen (N) source during aerobic growth by inducing an assimilatory nitrate reductase encoded by nasC located within the narK-bjgb-flp-nasC operon along with a nitrite reductase encoded by nirA at a different chromosomal locus. The global nitrogen two-component regulatory system NtrBC has been reported to coordinate the expression of key enzymes in nitrogen metabolism in several bacteria. In this study, we demonstrate that disruption of ntrC caused a growth defect in B. diazoefficiens cells in the presence of nitrate or nitrite as the sole N source and a decreased activity of the nitrate and nitrite reductase enzymes. Furthermore, the expression of narK-lacZ or nirA-lacZ transcriptional fusions was significantly reduced in the ntrC mutant after incubation under nitrate assimilation conditions. A B. diazoefficiens rpoN 1/2 mutant, lacking both copies of the gene encoding the alternative sigma factor σ54, was also defective in aerobic growth with nitrate as the N source as well as in nitrate and nitrite reductase expression. These results demonstrate that the NtrC regulator is required for expression of the B. diazoefficiens nasC and nirA genes and that the sigma factor RpoN is also involved in this regulation.




Similar content being viewed by others
Abbreviations
- Bjgb:
-
Bradyrhizobium japonicum haemoglobin
- BN3:
-
Bergersen minimal medium-nitrate
- C:
-
Carbon
- CFU:
-
Colony formation units
- Flp:
-
Flavoprotein
- MU:
-
Miller units
- MV-NiR:
-
Methyl viologen-dependent nitrite reductase
- MV-NR:
-
Methyl viologen-dependent nitrate reductase
- N:
-
Nitrogen
- NarK:
-
Nitrate/nitrite transporter
- NasC:
-
Assimilatory nitrate reductase
- NirA:
-
Assimilatory nitrite reductase
- NO:
-
Nitric oxide
- NtrB:
-
Two-component system kinase
- NtrC:
-
Two-component system response regulator
- OD500 :
-
Optical density-500 nm
- PSY:
-
Peptone–salts–yeast extract
- RpoN:
-
Alternative sigma factor
- WT:
-
Wild-type
- YEM:
-
Yeast-extract-mannitol
References
Bergersen FJ (1977) A treatise on dinitrogen fixation. In: Hardy RW, Silver W (eds) Biology: section III. Wiley, New York, pp 519–556
Cabello P, Roldán MD, Moreno-Vivián C (2004) Nitrate reduction and the nitrogen cycle in archaea. Microbiology 150:3527–3546. doi:10.1099/mic.0.27303-0
Cabrera JJ, Sanchez C, Gates AJ, Bedmar EJ, Mesa S, Richardson DJ, Delgado MJ (2011) The nitric oxide response in plant-associated endosymbiotic bacteria. Biochem Soc Trans 39:1880–1885. doi:10.1042/BST20110732
Cabrera JJ, Salas A, Torres MJ, Bedmar EJ, Richardson DJ, Gates AJ, Delgado MJ (2016) An integrated biochemical system for nitrate assimilation and nitric oxide detoxification in Bradyrhizobium japonicum. Biochem J 473:297–309. doi:10.1042/bj20150880
Chen P, Reitzer LJ (1995) Active contribution of two domains to cooperative DNA binding of the enhancer-binding protein nitrogen regulator I (NtrC) of Escherichia coli: stimulation by phosphorylation and the binding of ATP. J Bacteriol 177:2490–2496
Delgado MJ, Bonnard N, Tresierra-Ayala A, Bedmar EJ, Muller P (2003) The Bradyrhizobium japonicum napEDABC genes encoding the periplasmic nitrate reductase are essential for nitrate respiration. Microbiology 149:3395–3403
Drepper T, Wiethaus J, Giaourakis D, Groß S, Schubert B, Vogt M, Wiencek Y, McEwan AG, Masepohl B (2006) Cross-talk towards the response regulator NtrC controlling nitrogen metabolism in Rhodobacter capsulatus. FEMS Microbiol Lett 258:250–256. doi:10.1111/j.1574-6968.2006.00228.x
Evans CGT, Herbert D, Tempest DW (1970) The continuous cultivation of microorganisms. 2. Construction of a chemostat. Methods Microbiol 2:275–327
Forchhammer K (2008) PII signal transducers: novel functional and structural insights. Trends Microbiol 16:65–72. doi:10.1016/j.tim.2007.11.004
Franck WL, Qiu J, Lee H, Chang W, Stacey G (2015) DNA microarray-based identification of genes regulated by NtrC in Bradyrhizobium japonicum. Appl Environ Microbiol 81:5299–5308. doi:10.1128/aem.00609-15
Gates AJ, Luque-Almagro VM, Goddard AD, Ferguson SJ, Roldán MD, Richardson DJ (2011) A composite biochemical system for bacterial nitrate and nitrite assimilation as exemplified by Paracoccus denitrificans. Biochem J 435:743–753. doi:10.1042/bj20101920
Gutiérrez JC, Ramos F, Ortner L, Tortolero M (1995) nasST, two genes involved in the induction of the assimilatory nitrite—nitrate reductase operon (nasAB) of Azotobacter vinelandii. Mol Microbiol 18:579–591. doi:10.1111/j.1365-2958.1995.mmi_18030579.x
Ishida ML, Assumpção MC, Machado HB, Benelli EM, Souza EM, Pedrosa FO (2002) Identification and characterization of the two-component NtrY/NtrX regulatory system in Azospirillum brasilense. Braz J Med Biol Res 35:651–661
Jiang P, Ninfa AJ (1999) Regulation of autophosphorylation of Escherichia coli nitrogen regulator II by the PII signal transduction protein. J Bacteriol 181:1906–1911
Jiang P, Ninfa AJ (2009) α-Ketoglutarate controls the ability of the Escherichia coli PII signal transduction protein to regulate the activities of NRII (NtrB) but does not control the binding of PII to NRII. Biochemistry 48:11514–11521. doi:10.1021/bi901158h
Kirchner O, Tauch A (2003) Tools for genetic engineering in the amino acid-producing bacterium Corynebacterium glutamicum. J Biotechnol 104:287–299. doi:10.1016/S0168-1656(03)00148-2
Kullik I, Fritsche S, Knobel H, Sanjuan J, Hennecke H, Fischer HM (1991) Bradyrhizobium japonicum has two differentially regulated, functional homologs of the sigma 54 gene (rpoN). J Bacteriol 173:1125–1138
Leigh JA, Dodsworth JA (2007) Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol 61:349–377. doi:10.1146/annurev.micro.61.080706.093409
Li W, Lu C (2007) Regulation of carbon and nitrogen utilization by CbrAB and NtrBC Two-component systems in Pseudomonas aeruginosa. J Bacteriol 189:5413–5420. doi:10.1128/jb.00432-07
Lin JT, Stewart V (1998) Nitrate assimilation by bacteria. Adv Microb Physiol 39(1–30):379
Luque-Almagro VM, Gates AJ, Moreno-Vivián C, Ferguson SJ, Richardson DJ, Roldán MD (2011) Bacterial nitrate assimilation: gene distribution and regulation. Biochem Soc Trans 39:1838–1843. doi:10.1042/bst20110688
Luque-Almagro VM, Lyall VJ, Ferguson SJ, Roldan MD, Richardson DJ, Gates AJ (2013) Nitrogen oxyanion-dependent dissociation of a two-component complex that regulates bacterial nitrate assimilation. J Biol Chem 288:29692–29702. doi:10.1074/jbc.M113.459032
Martin GB, Chapman KA, Chelm BK (1988) Role of the Bradyrhizobium japonicum ntrC gene product in differential regulation of the glutamine synthetase II gene (glnII). J Bacteriol 170:5452–5459
Merrick MJ (1993) In a class of its own—the RNA polymerase sigma factor σ54(σN). Mol Microbiol 10:903–909. doi:10.1111/j.1365-2958.1993.tb00961.x
Merrick MJ, Edwards RA (1995) Nitrogen control in bacteria. Microbiol Rev 59:604–622
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, New York
Mongiardini EJ, Parisi GD, Quelas JI, Lodeiro AR (2016) The tight-adhesion proteins TadGEF of Bradyrhizobium diazoefficiens USDA 110 are involved in cell adhesion and infectivity on soybean roots. Microbiol Res 182:80–88
Moreno-Vivián C, Cabello P, Martínez-Luque M, Blasco R, Castillo F (1999) Prokaryotic nitrate reduction: molecular properties and functional distinction among bacterial nitrate reductases. J Bacteriol 181:6573–6584
Nicholas DJD, Nason A (1957) Determination of nitrate and nitrite. Methods Enzymol 3:981–984
Ninfa AJ, Reitzer LJ, Magasanik B (1987) Initiation of transcription at the bacterial glnAp2 promoter by purified E. coli components is facilitated by enhancers. Cell 50:1039–1046. doi:10.1016/0092-8674(87)90170-X
North AK, Klose KE, Stedman KM, Kustu S (1993) Prokaryotic enhancer-binding proteins reflect eukaryote-like modularity: the puzzle of nitrogen regulatory protein C. J Bacteriol 175:4267–4273
Ogawa K, Akagawa E, Yamane K, Sun ZW, LaCelle M, Zuber P, Nakano MM (1995) The nasB operon and nasA gene are required for nitrate/nitrite assimilation in Bacillus subtilis. J Bacteriol 177:1409–1413
Ohashi Y, Shi W, Takatani N, Aichi M, Maeda S, Watanabe S, Yoshikawa H, Omata T (2011) Regulation of nitrate assimilation in cyanobacteria. J Exp Bot 62:1411–1424. doi:10.1093/jxb/erq427
Patriarca EJ, Tatè R, Iaccarino M (2002) Key Role of Bacterial NH4 + metabolism in Rhizobium-plant symbiosis. Microbiol Mol Biol Rev 66:203–222. doi:10.1128/mmbr.66.2.203-222.2002
Pawlowski K, Klosse U, de Bruijn FJ (1991) Characterization of a novel Azorhizobium caulinodans ORS571 two-component regulatory system, NtrY/NtrX, involved in nitrogen fixation and metabolism. Mol Gen Genet MGG 231:124–138. doi:10.1007/bf00293830
Pino C, Olmo-Mira F, Cabello P, Martínez-Luque M, Castillo F, Roldán MD, Moreno-Vivián C (2006) The assimilatory nitrate reduction system of the phototrophic bacterium Rhodobacter capsulatus E1F1. Biochem Soc Trans 34:127–129. doi:10.1042/bst0340127
Pioszak AA, Jiang P, Ninfa AJ (2000) The Escherichia coli PII signal transduction protein regulates the activities of the two-component system transmitter protein NRII by direct interaction with the kinase domain of the transmitter module. Biochemistry 39:13450–13461. doi:10.1021/bi000795m
Regensburger B, Hennecke H (1983) RNA polymerase from Rhizobium japonicum. Arch Microbiol 135:103–109
Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57:155–176. doi:10.1146/annurev.micro.57.030502.090820
Reitzer LJ, Magasanik B (1985) Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci USA 82:1979–1983
Richardson JD, Berks CB, Russell AD, Spiro S, Taylor JC (2001) Functional, biochemical and genetic diversity of prokaryotic nitrate reductases. Cell Mol Life Sci CMLS 58:165–178. doi:10.1007/pl00000845
Romeo A, Sonnleitner E, Sorger-Domenigg T, Nakano MM, Eisenhaber B, Bläsi U (2012) Transcriptional regulation of nitrate assimilation in Pseudomonas aeruginosa occurs via transcriptional antitermination within the nirBD–PA1779–cobA operon. Microbiology 158:1543–1552. doi:10.1099/mic.0.053850-0
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, New York
Sánchez C, Cabrera JJ, Gates AJ, Bedmar EJ, Richardson DJ, Delgado MJ (2011) Nitric oxide detoxification in the rhizobia-legume symbiosis. Biochem Soc Trans 39:184–188. doi:10.1042/BST0390184
Schumacher J, Behrends V, Pan Z, Brown DR, Heydenreich F, Lewis MR, Bennett MH, Razzaghi B, Komorowski M, Barahona M, Stumpf MPH, Wigneshweraraj S, Bundy JG, Buck M (2013) Nitrogen and carbon status are integrated at the transcriptional level by the nitrogen regulator NtrC in vivo. MBio. doi:10.1128/mBio.00881-13
Shimizu K (2016) Metabolic regulation and coordination of the metabolism in bacteria in response to a variety of growth conditions. In: Ye Q, Bao J, Zhong J (eds) Bioreactor engineering research and industrial applications I: cell factories., Advances in biochemical engineering biotechnologySpringer, Berlin, pp 1–54
Simon R, Priefer U, Pühler A (1983) Vector plasmids for in vivo and in vitro manipulation of Gram-negative bacteria. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer, Heidelberg, pp 98–106
Stock AM, Robinson VL, Goudreau PN (2000) Two-component signal transduction. Annu Rev Biochem 69:183–215. doi:10.1146/annurev.biochem.69.1.183
Szeto WW, Nixon BT, Ronson CW, Ausubel FM (1987) Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activation of nitrogen fixation genes in free-living and symbiotic cells. J Bacteriol 169:1423–1432
van Heeswijk WC, Westerhoff HV, Boogerd FC (2013) Nitrogen assimilation in Escherichia coli: putting molecular data into a systems perspective. Microbiol Mol Biol Rev 77:628–695. doi:10.1128/mmbr.00025-13
Vieira J, Messing J (1982) The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259–268
Vincent JM (1974) Root-nodule symbioses with Rhizobium. In: Quispel A (ed) The biology of nitrogen fixation. American Elsevier Publishing Co., New York, pp 265–341
Wang B, Pierson LS, Rensing C, Gunatilaka MK, Kennedy CK (2012) NasT-mediated antitermination plays an essential role in the regulation of the assimilatory nitrate reductase operon in Azotobacter vinelandii. Appl Environ Microbiol 78:6558–6567. doi:10.1128/aem.01720-12
Weiss V, Claverie-Martin F, Magasanik B (1992) Phosphorylation of nitrogen regulator I of Escherichia coli induces strong cooperative binding to DNA essential for activation of transcription. Proc Natl Acad Sci USA 89:5088–5092
Wu SQ, Chai W, Lin JT, Stewart V (1999) General nitrogen regulation of nitrate assimilation regulatory gene nasR expression in Klebsiella oxytoca M5al. J Bacteriol 181:7274–7284
Zimmer DP, Soupene E, Lee HL, Wendisch VF, Khodursky AB, Peter BJ, Bender RA, Kustu S (2000) Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc Natl Acad Sci USA 97:14674–14679. doi:10.1073/pnas.97.26.14674
Acknowledgements
We thank Dr. Hans-Martin Fisher (ETH Zürich, Institute of Microbiology, Zürich, Switzerland) for kindly providing the B. japonicum rpoN 1/2 mutant. We are also grateful to Paula Giménez, Silvana Tongiani, Abel Bortolameotti (members of CPA CONICET at IBBM), Ruben Bustos from UNLP, and Alba Hidalgo-García for their excellent technical assistance (EEZ CSIC). Dr. Donald F. Haggerty edited the final version of the manuscript. This work was supported by the Agencia Nacional de Promoción de la Investigación Científica y Tecnológica (ANPCyT) project PICT 2013-2864, Consejo Nacional de Investigaciones Científicas y Técnicas—CONICET and Secyt-UNLP, Argentina. MJD received financial support from the European Regional Development Fund (ERDF) cofinanced Grants AGL2013-45087-R from the Ministerio de Economía y Competitividad (Spain) and PE2012-AGR1968 from the Junta de Andalucía. Continuous support from Junta de Andalucía to group BIO275 is also acknowledged. MFL was supported by fellowships from CONICET and by a short-stay fellowship from EMBO. SLLG is researcher at CONICET.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
López, M.F., Cabrera, J.J., Salas, A. et al. Dissecting the role of NtrC and RpoN in the expression of assimilatory nitrate and nitrite reductases in Bradyrhizobium diazoefficiens . Antonie van Leeuwenhoek 110, 531–542 (2017). https://doi.org/10.1007/s10482-016-0821-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0821-3