Abstract
Complex agroforestry systems are suggested as a possible solution to reduce the effects of deforestation in the tropics while enhancing the livelihoods of local human populations. Coffee (Coffea spp.) is one of the most important commodity crops in the world that can easily be cultivated in complex agroforestry systems. Coffee agroforestry systems usually sustain higher biodiversity levels than sun-exposed fields while keeping similar levels of productivity considering the several benefits of growing coffee under a complex system. We aim to explore the richness and abundance of invertebrates in coffee home gardens in West Java, Indonesia by comparing 14 sun-exposed and 14 shade-grown gardens. We collected data in March/April 2019 via pitfall traps, pan traps, and beating tray in each field. We ran generalised linear models to assess whether the number of species and the number of individuals of insects differed between sun-exposed vs. shade-grown coffee gardens, and tested associations between main taxa. Overall, there was no difference in the richness (sun-exposed: 19.86 ± SE1.19; shade-grown: 19.71 ± SE1.19; Z-value = 0.12, p value = 0.904) and abundance (sun-exposed: 141.93 ± SE 3.18; shade-grown: 139.93 ± SE3.16; Z-value = 0.35, p value = 0.706) of invertebrates in coffee gardens, although taxa specific differences were present. Sun-exposed fields had a higher abundance of invertebrates considered as pests (Blattodea: Rhinotermitidae, Ectobiidae; Coleoptera: Scarabaeidae, Lycidae and Tenebrionidae; Diptera: Anisolabididae, Drosophilidae and Sarcophagidae). Camponotus spp. were the most dominant ants in shade-grown gardens while Dolichoderus spp. and Myrmicaria spp. were more abundant in sun-exposed gardens. Despite the fact that sun-exposed coffee fields registered higher abundance of invertebrate pests than shade-grown coffee fields, the richness of invertebrates did not substantially vary between sun-exposed and shade-grown coffee, suggesting that the matrix of gardens offers advanced ecosystem services. It is important to keep the complexity of agroforestry systems that provide key habitats for biodiversity.
Similar content being viewed by others
Introduction
Tropical forests are heavily impacted by the increased need for food production and the consequent expansion of agricultural land (Foley et al. 2011). The complexity of agricultural systems, in terms of ecological and crop diversity, are also reduced, with intensive monocultures becoming more abundant than complex agroforestry systems, to meet the high food demand (Laurence et al. 2014). Agroforestry systems (i.e., complexes of crops and shade trees) are suggested as a possible solution to reduce the effects of deforestation of tropical forests, while also enhancing the livelihoods of local communities (Bhagwat et al. 2008). Agroforestry systems can sustain similar biodiversity of natural habitats for some taxa (Bhagwat et al. 2008; De Beenhouwer et al. 2013; Santos-Heredia et al. 2018) and can partially restore the soil structure after conversion of forests to monocultures (Saputra et al. 2020). They are, however, likely to conserve little functional diversity unless the adjacent natural habitat is also protected (Cannon et al. 2019). Thus, land sparing agriculture (i.e., intensification of existing farmlands and expansion of protected natural habitats) is often preferred to land sharing agriculture (i.e., agroforestry systems) (Cannon et al. 2019). To show the conservation potential of land sharing system, more data are needed, especially in the tropics since some regions of high biodiversity outside the Neotropics are less studied.
Coffee (Coffea spp.) is one of the most important commodity crops in the world (DaMatta et al. 2019), with around 25 million people estimated to depend on its production for their livelihoods (Bunn et al. 2015). Coffee, together with cocoa (Theobroma cacao), are the best examples of crops that can easily be cultivated in complex agroforestry systems. Traditionally cultivated under dense and diverse shade canopy (Moguel and Toledo 1999), these crops can sustain high biodiversity of various taxa when under shade trees (e.g., invertebrates: Armbrecht and Perfecto 2003; Perfecto et al. 2003; Borkhataria et al. 2012; birds: Perfecto et al. 2003; Gordon et al. 2007; Borkhataria et al. 2012; Philpott and Bichier 2012; mammals: Gordon et al. 2007; Caudill et al. 2015). The presence of shade trees in coffee agroforestry systems can also enhance carbon sequestration, drought resistance, functional biodiversity, soil fertility, as well as provide natural solutions for weed and biological pest control (reviewed in Tscharntke et al. 2011). In addition, coffee agroforestry systems show smaller temperature fluctuations (López-Bravo et al. 2012; Mariño et al. 2016), improve microclimate conditions and deep-water drainage (De Carvalho et al. 2021), and can provide key resources for wildlife (Perfecto et al. 1996) when compared to sun-exposed coffee fields.
Several taxa of insects are considered as bioindicators for assessing the state of agroforestry systems (Kevan 1999; Andersen et al. 2002; Jimenez-Soto et al. 2019). Many insects are known to be sensitive to land use changes despite their important roles in the ecosystem as pollinators, pest control agents and nutrient cyclers (Peck et al. 1998; Rainio and Niemela 2003; Losey and Vaughan 2006). For example, ants (Formicidae) can be important biological control agents in coffee fields as they predate the main coffee pest borer, Hypothenemus hampei, a beetle (Family Curculionidae) that severely damages coffee seeds (Morris et al. 2018; Jimenez-Soto et al. 2019). Soil insects such as ants and termites (Isoptera) can move particles in the soils and favour the mobilisation of nutrients (Dangles et al. 2012) as well as hasten organic matter decomposition (Crespo-Pérez et al. 2020). Pollinators from different orders (e.g., Lepidoptera, Hymenoptera, and Diptera) are important bioindicators as most of the crops benefit from cross-pollination by producing more fruits, increasing fruit quality and maintaining high genetic variability (Klein et al. 2007; Garibaldi et al. 2011).
The question about changes in invertebrate richness and abundance between different agroforestry systems remains elusive. Ant species richness and abundance, for example, is higher either in shade-grown coffee (Armbrecht and Perfecto 2003; Perfecto et al. 2003) or in sun-exposed coffee (Arenas-Clavijo and Armbrecht 2019). Borkhataria et al. (2012) found higher abundance of Coleoptera, Diptera, Hemiptera, Hymenoptera, and Lepidoptera in shade-grown than in sun-exposed coffee fields and a higher abundance of Orthoptera in sun-exposed coffee fields. Pollinators are expected to be more abundant in shade-grown coffee as flowers from other plant species can attract them, but the abundance of some species do not differ between sun-exposed and shade-grown coffee (e.g., bees, Classen et al. 2014). Moreover, Prado et al. (2021) found no influence of flower availability on shade trees or type of coffee field (shade vs. sun-exposed) on the frequency of bee visitation. The response is therefore taxon specific, with the need to consider regional and environmental variables (Smith et al. 2015).
We aimed to explore the richness and abundance of invertebrates in coffee home gardens in West Java, Indonesia by comparing sun-exposed and shade-grown gardens. Indonesia is one of the 17 megadiverse countries in the World and a biodiversity hotspot, with many endemic species under a constant threat of extinction (von Rintelen et al. 2017). The study area is a good model to test the effect of sun vs. shade home gardens without the influence of environmental variables as it is subject to dry periods of around 5 months but still maintains similar ambient temperatures. In addition, there are relatively few studies on the impact of different management practices on coffee fields in Indonesia, despite being the fourth largest coffee producer in the world (Szenthe 2020). Despite evidence of regional differences in animal diversity between sun and shade coffee fields from neotropical studies, few researchers have investigated this in the Asian context, including Indonesia. Coffee production in Indonesia has increased since 2010, both in terms of local and exported coffee markets (Prajanti et al. 2020). With the increase in these markets, we expect an intensification in the management of coffee fields and a consequent increase in sun exposed fields. It is therefore key to understand the implications of shifting cultivation to sun fields in this biodiversity hotspot. We predict an overall decrease in richness and abundance of invertebrates in sun-exposed coffee, compared with shade-grown coffee, although we expect taxon specific differences on the response. We predict that shade-grown coffee would favour the presence and abundance of invertebrates with important ecological roles for the ecosystem, while sun-exposed coffee fields would host higher abundances of invertebrates typically regarded as pests.
Materials and methods
Study area
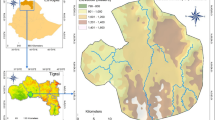
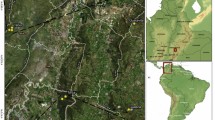
We collected data in 28 coffee home gardens in the municipalities of Cipaganti and Pangauban, West Java, Indonesia (7.2786°S, 107.7577°E; elevation ~ 1350 m a.s.l.; Fig. 1). The area consists of an agroforestry system of interconnected crops usually separated by trees alongside trees planted inside gardens (Nekaris et al. 2017; Campera et al. 2021b). This agroforestry system lies in the foothills of Mount Puntang, which is part of the mountain range containing the active volcano Mount Papandayan. The forests on Mount Papandayan are protected in part as a watershed (hutan lindung) and in part to protect its unique biodiversity (cagar alam), but the agroforestry system is not included in the protected area. The climate is ever-wet, with a mean annual precipitation exceeding 2500 mm and a drier period between April and September (Nekaris et al. 2017). In addition to coffee (Coffea arabica), there are other yielding crops such as tea (Camellia sinensis), chayote (Sechium edule), carrot (Daucus carota), white cabbage (Pieris brassicae), tomato (Solanum lycopersicum), cassava (Manihot esculenta), and potato (Solanum tuberosum). The agroforestry systems is on a mixture of clay soil and sandy soil.
Data collection
Out of the estimated 400 small-holder coffee gardens present in the area, we sampled only a subset of gardens randomly chosen to have an equal number of sun-exposed and shade-grown gardens. Coffee gardens surveyed covered a mean area of 1,216 ± SD 877 m2, for a total of 34,055 m2. The sampled coffee gardens were at a distance of 1734 ± SD 371 m (range = 1296–2505 m) from the edge of the continuous forest from which they were connected by a series of home gardens and bamboo forest patches. We divided coffee gardens into sun vs. shade depending on the shade cover and the variety of shade trees. To determine shade cover, we used the Canopeo App, which calculates the proportion of area shaded from photographs (Patrignani and Ochsner 2015). We took four random and independent photographs and calculated the mean value for each coffee garden. The minimum distance between points of photo capture was 10 m, and the minimum distance from field edge was 5 m. We ensured that the photos did not include understory canopy such as banana leaves, which would have biased the calculation of the tree shade cover. We recorded the tree species that were used as shade trees in the sampled gardens. The main shade trees in coffee gardens were Grevillea robusta, Maesopsis eminii, Manglietia glauca, Melaleuca cajuputi, Persea americana, and Toona sureni. We divided sun-exposed gardens (n = 14) and shade-grown gardens (n = 14). We recorded the temperature and humidity via a weather station (HOBO RX3000) in proximity to the gardens to represent the general weather conditions during the data collection period and not the weather conditions at the garden per se.
We collected data during the beginning of the dry period (March/April 2019) at times where there was no rain or wind that could have affected the presence of invertebrates. The mean temperature during the data collection varied little (mean = 21.4 ± SD 0.8 °C). We installed the traps between 0600 and 0700 h in a single 12.25 m2 plot in the middle of each coffee garden. We installed six pitfall traps (diameter: 11 cm) set along the diagonal keeping 1 m spacing between traps and positioned pan traps (inner dimensions: 37 × 27 × 2 cm) in opposite corners of the plot at a height of one meter (Fig. 2) (Woodcock 2005). We used two colours (blue and yellow) for pan traps as there can be a colour preference in some taxa of pollinators (Heneberg and Bogusch 2014). We filled pitfalls to one third and pan traps to the brim with a solution of clean water and a few drops of detergent. After installing the traps, we used the beating tray method on three random coffee plants in the garden. We placed a white tray of 1 m2 below the plant, and then shook the whole plant for 5 s and collected all the insects dropped on the tray using an insect aspirator (Ozanne 2005). We then left the garden and came back 6 h after installing the traps to collect the samples. We substituted the solution of water and detergent with 70% ethanol and put in the freezer to preserve the samples and allow subsequent counting and identification. The identification was done to the genus level when possible, and we considered morphospecies. We used taxonomic keys and Olympus SZ61 microscope to perform the identification. For an easier representation of the results, we first present the data grouped by Order and then make additional separations within the main orders detected. For the Formicidae, we present the results at genus level as they were the most abundant taxa.
Data analysis
We ran generalised linear models to assess whether the number of species and the number of individuals of insects differed between sun-exposed vs. shade-grown coffee gardens. We fit the dependent variables with Poisson distributions for count data. We additionally ran generalised linear models considering each Family separately (and genera of the Family Formicidae) for the families for which we found at least ten individuals to better understand the differences between taxa. We used the function “glm” in R 4.0.4 for the analysis. We considered p = 0.05 as threshold level for significance. For the species that were present in at least ten gardens, we run a correlation matrix via “cor.mest” function in the package “corrplot” and corrected p-value via Bonferroni-Holm method in “p.adjust” function (Wei and Simko 2021).
Results
Sun-exposed gardens had a shade cover < 10% and with shade mainly given by trees at the border of gardens. Shade-grown gardens had a shade cover > 15% and had higher richness of shade trees in the middle of the coffee gardens (Table 1). The other parameters relative to the coffee gardens and the data collection did not change between sun-exposed and shade-grown gardens.
In total, we recorded 3,962 individuals from 111 species of invertebrates in coffee gardens (Table 2). With the pitfall trap method, we collected the highest abundance of invertebrates (3,114 specimens from 49 species), although the beating tray method resulted in the highest number of species (538 specimens from 66 species). The pan traps collected only 211 specimens from 22 species (yellow) and 99 specimens from 18 species (blue) but were more efficient in collecting data on Hymenoptera not belonging to the Family Formicidae, and on Diptera.
The total number of species of invertebrates did not vary significantly between sun-exposed and shade-grown coffee gardens (Z-value = 0.12, p value = 0.904; Table 3). The overall abundance of invertebrates also did not vary (Z-value = 0.35, p value = 0.706), but significant differences occurred at the Order level. The abundance of Blattodea (Z-value = 8.69, p value < 0.001) and Diptera (Z-value = 3.78, p value < 0.001) was significantly higher in sun-exposed gardens, while the abundance of Orthoptera was significantly higher in shade-grown gardens (Z-value = −4.05, p value < 0.001). When investigating differences at Family levels, we found additional significant results. Within the Araneae, the individuals of the Family Lycosidae were more abundant in shade-grown gardens (Z-value = − 2.30, p value = 0.021), while Linyphiidae were more abundant in sun-exposed gardens (Z-value = −2.78, p value = 0.005) (Fig. 3). Within the Blattodea, Rhinotermitidae (Z-value = 8.12, p value < 0.001) and Ectobiidae (Z-value = 2.59, p value = 0.010) were more abundant in sun-exposed gardens. Coleoptera of the Families Scarabaeidae (Z-value = 2.46, p-value = 0.014), Lycidae (Z-value = 2.22, p-value = 0.026) and Tenebrionidae (Z-value = 1.97, p value = 0.049), and Diptera of the families Culicidae (Z-value = 2.31, p value = 0.021), Sarcophagidae (Z-value = 2.91, p value = 0.004) and Drosophilidae (Z-value = 1.96, p value = 0.050) were more abundant in sun-exposed gardens. Within the Family Formicidae, we found variability among genera with higher abundance of Camponotus spp. (Z-value = -10.14, p-value < 0.001) in shade-grown gardens and higher abundance of Myrmicaria spp. (Z-value = 10.10, p value < 0.001) and Dolichoderus spp. (Z-value = 5.17, p value < 0.001) in sun-exposed gardens. The abundance of Camponotus spp. was negatively correlated to the abundance of Dolichoderus spp. (r = − 0.53, p = 0.003) and Myrmicaria spp. (r = − 0.53, p = 0.004) (Fig. 4). The abundance of Paratrechina spp. was positively correlated to the abundance of Zodariidae (Araneae) (r = 0.53, p = 0.004), Chelisochidae (Dermaptera) (r = 0.60, p < 0.001), and Gryllidae (Orthoptera) (r = 0.59, p < 0.001). We also found a positive correlation between the abundance of Rhinotermitidae (Blattodea) and Sarcophagidae (Diptera) (r = 0.62, p < 0.001) and between the abundance of Anisolabididae (Dermaptera) and Drosophilidae (Diptera) (r = 0.57, p = 0.002).
Abundance of invertebrates in 28 coffee home gardens (14 sun-exposed and 14 shade-grown) in Indonesia. Plots show raw data represented via box plots embedded in violin plots. A: Araneae; B: Blattodea; C: Coleoptera; D: Dermaptera; E: Diptera; F: Formicidae; G: other Hymenoptera; H: Orthoptera. *p < 0.05; **p < 0.01 based on generalised linear models. The colour of the * indicates whether values are significantly higher in sun-exposed (yellow) or shade-grown (green) gardens. A: Araneae; B: Blattodea; C: Coleoptera; D: Dermaptera: E: Diptera; F: Formicidae; G: Hymenoptera (other); H: Orthoptera. (Color figure online)
Discussion
We found that the complexity of coffee home gardens, evaluated by comparing sun-exposed and shade-grown gardens, influenced the abundance of several taxa of invertebrates, although overall there was no difference in terms of richness and abundance of invertebrates between the two types of gardens. This finding can be dependent on the fact that the sun-exposed home gardens in the study area were included in a matrix of other home gardens with shade cover. Despite this factor, the abundance of several taxa that can be considered as pests were still more abundant in sun-exposed fields. Termites of the Family Rhinotermitidae, for example, are known to cause severe damage to mature trees and can bring about a reduction in crop productivity (Cowie et al. 1989; Fajar et al. 2021). Cockroaches of the Family Ectobiidae (Blattella sp. in the study site) are omnivores and can damage crops by eating leaves and predating on eggs of Lepidoptera and Hemiptera (Capinera 2020). Beetles of Families Lycidae (Cautires sp. and Scarelus sp. in the study site) and Tenebrionidae (Euhemicera sp. in the study site) also eat leaves and are predators of larvae (Lundgren et al. 2014; Capinera 2020), although they can bring benefits to coffee production as they are occasional pollinators of coffee flowers (Hipólito et al. 2018) and are agents of organic matter decomposition (Crespo-Pérez et al. 2020). Sun-exposed gardens also had a higher abundance of parasites from the Family Diptera. Shade-grown gardens had a higher abundance of Orthoptera, and this is somehow unexpected since Orthoptera are expected to prefer areas with bare ground used for basking and oviposition (Fartmann et al. 2012; Rahman et al. 2012), however, also found a higher abundance of Orthoptera in agroforestry systems than in monocultures. Considering additional factors such as diversity of other crops and ground vegetation can help better understand this pattern.
We revealed some interesting associations between taxa. In ant communities, the dominant genera were Camponotus spp. in shade-grown gardens and Dolichoderus spp. and Myrmicaria spp. in sun-exposed gardens. This pattern of exclusion of dominant species in relation to the complexity of the coffee field was also found in Puerto Rico (Perfecto and Vandermeer 2020). Paratrechina spp., a predator of several invertebrate larvae that are both pests and important pollinators (Romeis et al. 1995; Silva et al. 2015), were associated with the ant mimicking spiders from the Family Zodariidae, suggesting that the detected species of Zodariidae were highly specialised on hunting Paratrechina spp. (Pekár et al. 2018). Anisolabididae was positively associated with Drosophilidae, suggesting that the detected earwigs are predators of the larvae of flies (Garcia et al. 2020). Eggs or larvae of Gryllidae might also be target of predator spiders and earwigs (Midega et al. 2004), although we would need more experimental support to draw conclusions on the associations revealed via the correlation matrix.
There are a series of limitations and additional considerations based on our results. First, there are additional predictors to invertebrate abundance that were not considered in our investigation. For example, invertebrate abundance and richness can be shaped by the use of agrochemicals (Iwasaki and Hoogendorn 2021; Manson et al. 2022), shade tree richness (Campera et al. 2021a), soil moisture content (Staley et al. 2007), fire (Swengel 2001), and climate change (Barnett and Facey 2016). Second, the methods used were mainly effective in detecting ground-dwelling invertebrates, although pan traps and beating trays were effective in detecting other taxa. Given the methods used, the results are probably more accurate for some taxa (e.g., Araneae, Blattodea, Coleoptera, Formicidae) and less accurate for other taxa that require additional methods for data collection (e.g., Hemiptera, Hymenoptera not Formicidae, Lepidoptera, Odonata). For example, pan traps are commonly used to assess the communities of flower-visiting insects in an area (Heneberg and Bogusch 2014), but other methods (e.g., net sampling) can perform better or be complementary (Popic et al. 2013). The reduced number of flower-visiting insects might also be due to the temporal limitation of our sampling that was not done in the peak of the flowering season in coffee gardens (usually July-August), although other plants were flowering at the time of data collection. These methodological issues, however, do not limit the importance of our findings since our scope is to present the variations in invertebrate abundance and richness between sun-exposed and shade-grown gardens.
Our findings suggest that sun-exposed coffee fields host a higher abundance of invertebrate pests than shade-grown coffee fields. Still, the abundance of functional invertebrates such as ants and spiders were very high compared to the abundance of pests. In addition, the diversity of invertebrates was not different between the two field types, suggesting the ecosystem services offered by the agroforestry environment are efficient. The agroforestry environment is particularly important as it is an important habitat for the conservation of threatened mammal and bird species (Nekaris et al. 2020; Campera et al. 2021b). Coffee farmers in the area mainly use organic practices, favoured by extensive training for organic fertilisers and pesticides and conservation education initiatives (Campera et al. 2021b). We also promoted the planting of shade tree saplings (~ 30,000) in the circa 400 coffee gardens present in the study area that could provide long-term benefits (Campera et al. 2021b), considering the increase in the coffee market in Indonesia (Prajanti et al. 2020) and the possible intensification in crop management in the near future (Schroth et al. 2015). Accordingly, a land sharing agroforestry environment can also enhance the credibility of sustainable coffee production that is wildlife-friendly and contributes to the food security of local communities (Duffy et al. 2021). This is also potentially important to consumers seeking sustainable brands that are authentic in their conservation actions. Considering the constant decline of terrestrial invertebrates (van Klink et al. 2020), birds and mammals (Spooner et al. 2018), the efforts in conservation should focus on maintaining and promoting complex agroforestry systems that might represent the future of conservation for some threatened species that prefer human-modified habitats.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Andersen AN, Hoffmann BD, Müller WJ, Griffiths AD (2002) Using ants as bioindicator in land management: simplifying assessment of ant community responses. J Appl Ecol 39:8–17. https://doi.org/10.1046/j.1365-2664.2002.00704.x
Arenas-Clavijo A, Armbrecht I (2019) Soil ants (Hymenoptera: Formicidae) and ground beetles (Coleoptera: Carabidae) in a coffee agroforestry landscape during a severe-drought period. Agroforest Syst 93:1781–1792. https://doi.org/10.1007/s10457-018-0283-x
Armbrecht I, Perfecto I (2003) Litter-twig dwelling ant species richness and predation potential within a forest fragment and neighboring coffee plantations of contrasting habitat quality in Mexico. Agric Ecosyst Environ 97:107–115. https://doi.org/10.1016/S0167-8809(03)00128-2
Barnett KL, Facey SL (2016) Grasslands, invertebrates, and precipitation: a review of the effects of climate change. Front Plant Sci 7:1196. https://doi.org/10.3389/fpls.2016.01196
Bhagwat SA, Willis KJ, Birks HJB, Whittaker RJ (2008) Agroforestry: a refuge for tropical biodiversity? Trends Ecol Evol 23:261–267. https://doi.org/10.1016/j.tree.2008.01.005
Borkhataria RR, Collazo JA, Groom MJ (2012) Species abundance and potential biological control services in shade vs. sun coffee in Puerto Rico. Agric Ecosyst Environ 151:1–5. https://doi.org/10.1016/j.agee.2012.01.025
Bunn C, Läderach P, Ovalle Rivera O, Kirschke D (2015) A bitter cup: climate change profile of global production of Arabica and Robusta coffee. Clim Change 129:89–101. https://doi.org/10.1007/s10584-014-1306-x
Campera M, Balestri M, Manson S, Hedger K, Ahmad N, Nijman V, Budiadi B, Imron MA, Nekaris KAI (2021a) Shade trees and agrochemical use affect butterfly assemblages in coffee home gardens. Agric Ecosyst Environ 319:107547. https://doi.org/10.1016/j.agee.2021.107547
Campera M, Budiadi B, Adinda E, Ahmad N, Balestri M, Hedger K, Imron MA, Manson S, Nijman V, Nekaris KAI (2021b) Fostering a wildlife-friendly program for sustainable coffee farming: the case of small-holder farmers in Indonesia. Land 10:121. https://doi.org/10.3390/land10020121
Cannon PG, Gilroy JJ, Tobias JA, Anderson A, Haugaasen T, Edwards DP (2019) Land-sparing agriculture sustains higher levels of avian functional diversity than land sharing. Glob Chang Biol 25:1576–1590. https://doi.org/10.1111/gcb.14601
Capinera J (2020) Handbook of vegetable pests, 2nd edn. Elsevier, Amsterdam
Caudill SA, DeClerck FJA, Husband TP (2015) Connecting sustainable agriculture and wildlife conservation: does shade coffee provide habitat for mammals? Agric Ecosyst Environ 199:85–93. https://doi.org/10.1016/j.agee.2014.08.023
Classen A, Peters MK, Ferger SW, Helbig-Bonitz M, Schmack JM, Maasen G, Schleuning M, Kalko EKV, Böhning-Gaese K, Steffan-Dewenter I (2014) Complementary ecosystem services provided by pest predators and pollinators increase quantity and quality of coffee yields. Proc Biol Sci 281:20133148. https://doi.org/10.1098/rspb.2013.3148
Cowie RH, Logan JWM, Wood TG (1989) Termite (Isoptera) damage and control in tropical forestry with special reference to Africa and Indo-Malaysia: a review. Bull Entomol Res 79:173–184. https://doi.org/10.1017/S0007485300018150
Crespo-Pérez V, Kazakou E, Roubik DW, Cárdenas RE (2020) The importance of insects on land and in water: a tropical view. Curr Opin Insect Sci 40:31–38. https://doi.org/10.1016/j.cois.2020.05.016
DaMatta FM, Rahn E, Läderach P, Ghini R, Ramalho JC (2019) Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim Change 152:167–178. https://doi.org/10.1007/s10584-018-2346-4
Dangles O, Carpio C, Woodward G (2012) Size-dependent species removal impairs ecosystem functioning in a large-scale tropical field experiment. Ecology 93:2615–2625. https://doi.org/10.1890/12-0510.1
De Beenhouwer M, Aerts R, Honnaya O (2013) A global meta-analysis of the biodiversity and ecosystem service benefits of coffee and cacao agroforestry. Agric Ecosyst Environ 175:1–7. https://doi.org/10.1016/j.agee.2013.05.003
de Carvalho AF, Fernandes-Filho EI, Daher M, de Carvalho Gomes L, Cardoso IM, Alves Fernandes RB, Schaefer CEGR (2021) Microclimate and soil and water loss in shaded and unshaded agroforestry coffee systems. Agroforest Syst 95:119–134. https://doi.org/10.1007/s10457-020-00567-6
Duffy C, Toth GG, Hagan RPO, Mckeown PC, Rahman SA, Widyaningsih Y, Sunderland TCH, Spillane C (2021) Agroforestry contributions to smallholder farmer food security in Indonesia. Agroforest Syst. https://doi.org/10.1007/s10457-021-00632-8
Fajar A, Himmi SK, Latif A, Tarmadi D, Kartika T, Guswenrivo I, Yusuf S, Yoshimura T (2021) Termite assemblage and damage on tree trunks in fast-growing teak plantations of different age: a case study in West Java, Indonesia. Insects 12:295. https://doi.org/10.3390/insects12040295
Fartmann T, Kramer B, Stelzner F, Poniatowski D (2012) Orthoptera as ecological indicators for succession in steppe grassland. Ecol Indic 20:337–344. https://doi.org/10.1016/j.ecolind.2012.03.002
Foley JA, Ramankutty N, Brauman KA et al (2011) Solutions for a cultivated planet. Nature 478:337–342. https://doi.org/10.1038/nature10452
Garcia FRM, Ovruski SM, Suárez L, Cancino J, Liburd OE (2020) Biological control of tephritid fruit flies in the Americas and Hawaii: a review of the use of parasitoids and predators. Insects 11:662. https://doi.org/10.3390/insects11100662
Garibaldi LA, Steffan-Dewenter I, Kremen C et al (2011) Stability of pollination services decreases with isolation from natural areas despite honey bee visits: habitat isolation and pollination stability. Ecol Lett 14:1062–1072. https://doi.org/10.1111/j.1461-0248.2011.01669.x
Gordon C, Manson R, Sundberg J, Cruz-Angon A (2007) Biodiversity, profitability, and vegetation structure in a Mexican coffee agroecosystem. Agric Ecosyst Environ 118:256–266. https://doi.org/10.1016/j.agee.2006.05.023
Heneberg P, Bogusch P (2014) To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera? J Insect Conserv 18:1123–1136. https://doi.org/10.1007/s10841-014-9723-8
Hipólito J, Boscolo D, Viana BF (2018) Landscape and crop management strategies to conserve pollination services and increase yields in tropical coffee farms. Agric Ecosyst Environ 256:218–225. https://doi.org/10.1016/j.agee.2017.09.038
Iwasaki JM, Hoogendorn K (2021) Non-insecticide pesticide impacts on bees: a review of methods and reported outcomes. Agric Ecosyst Environ 314:107423. https://doi.org/10.1016/j.agee.2021.107423
Jimenez-Soto E, Morris JR, Letourneau DK, Philpott SM (2019) Vegetation connectivity increases ant activity and potential for ant-provided biocontrol services in a tropical agroforest. Biotropica 51:50–61. https://doi.org/10.1111/btp.12616
Kevan PG (1999) Pollinators as bioindicators of the state of the environment: species, activity and diversity. Agric Ecosyst Environ 74:373–393. https://doi.org/10.1016/S0167-8809(99)00044-4
Klein A-M, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc B 274:303–313. https://doi.org/10.1098/rspb.2006.3721
Laurance WF, Sayer J, Cassman KG (2014) Agricultural expansion and its impacts on tropical nature. Trends Ecol Evol 29:107–116. https://doi.org/10.1016/j.tree.2013.12.001
López-Bravo DF, Virginio-Filho EDM, Avelino J (2012) Shade is conducive to coffee rust as compared to full sun exposure under standardized fruit load conditions. Crop Prot 38:21–29. https://doi.org/10.1016/j.cropro.2012.03.011
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311. https://doi.org/10.1641/0006-3568(2006)56[311:TEVOES]2.0.CO;2
Lundgren JG, López-Lavalle LAB, Parsa S, Wyckhuys KAG (2014) Molecular determination of the predator community of a cassava whitefly in Colombia: pest-specific primer development and field validation. J Pest Sci 87:125–131. https://doi.org/10.1007/s10340-013-0509-7
Manson S, Nekaris KAI, Hedger K, Balestri M, Ahmad N, Adinda E, Budiadi B, Imron MA, Nijman V, Campera M (2022) Flower visitation time and number of visitor species are reduced by the use of agrochemicals in coffee home gardens. Agronomy 12:509. https://doi.org/10.3390/agronomy12020509
Mariño YA, Pérez ME, Gallardo F, Trifilio M, Cruz M, Bayman P (2016) Sun vs. shade affects infestation, total population and sex ratio of the coffee berry borer (Hypothenemus hampei) in Puerto Rico. Agric Ecosyst Environ 222:258–266. https://doi.org/10.1016/j.agee.2015.12.031
Midega CAO, Ogol CKPO, Overholt WA (2004) Effect of agroecosystem diversity on natural enemies of maize stemborers in coastal Kenya. Int J Trop Insect Sci 24:280–286. https://doi.org/10.1079/IJT200441
Moguel P, Toledo VM (1999) Biodiversity conservation in traditional coffee systems of Mexico. Conserv Biol 13:11–21. https://doi.org/10.1046/j.1523-1739.1999.97153.x
Morris JR, Jimenez-Soto ME, Philpott SM, Perfecto I (2018) Ant-mediated biological control of the coffee berry borer (Hypothenemus hampei Ferrari): diversity, ecological complexity, and conservation biocontrol. Myrmecol News 26:1–17. https://doi.org/10.1016/j.biocontrol.2019.05.011
Nekaris KAI, Poindexter S, Reinhardt KD, Sigaud M, Cabana F, Wirdateti W, Nijman V (2017) Coexistence between Javan slow lorises (Nycticebus javanicus) and humans in a dynamic agroforestry landscape in West Java, Indonesia. Int J Primatol 38:303–320. https://doi.org/10.1007/s10764-017-9960-2
Nekaris KAI, Handby V, Campera M, Birot H, Hedger K, Eaton J, Imron MA (2020) Implementing and monitoring the use of artificial canopy bridges by mammals and birds in an Indonesian agroforestry environment. Diversity 12:399. https://doi.org/10.3390/d12100399
Ozanne CMP (2005) Sampling methods for forest understory vegetation. In: Leather SR (ed) Insect sampling in forest ecosystems. Blackwell Publishing, Oxford, pp 58–76
Patrignani A, Ochsner TE (2015) Canopeo: a powerful new tool for measuring fractional green canopy cover. Agron J 107:2312–2320. https://doi.org/10.2134/agronj15.0150
Peck SL, McQuaid B, Campbell CL (1998) Using ant species (Hymenoptera: Formicidae) as a biological indicator of agroecosystem condition. Environ Entomol 27:1102–1110. https://doi.org/10.1093/ee/27.5.1102
Pekár S, Petráková L, Šedo O, Korenko S, Zdráhal Z (2018) Trophic niche, capture efficiency and venom profiles of six sympatric ant-eating spider species (Araneae: Zodariidae). Mol Ecol 27:1053–1064. https://doi.org/10.1111/mec.14485
Perfecto I, Vandermeer J (2020) The assembly and importance of a novel ecosystem: the ant community of coffee farms in Puerto Rico. Ecol Evol 10:12650–12662. https://doi.org/10.1002/ece3.6785
Perfecto I, Rice RA, Greenberg R, VanderVoort ME (1996) Shade coffee: a disappearing refuge for biodiversity. Bioscience 46:598–608. https://doi.org/10.2307/1312989
Perfecto I, Mas A, Dietsch T, Vandermeer J (2003) Conservation of biodiversity in coffee agroecosystems: a tri-taxa comparison in southern Mexico. Biodivers Conserv 12:1239–1252. https://doi.org/10.1023/A:1023039921916
Philpott SM, Bichier P (2012) Effects of shade tree removal on birds in coffee agroecosystems in Chiapas, Mexico. Agric Ecosyst Environ 149:171–180. https://doi.org/10.1016/j.agee.2011.02.015
Popic TJ, Davila YC, Wardle GM (2013) Evaluation of common methods for sampling invertebrate pollinator assemblages: net sampling out-perform pan traps. PLoS ONE 8:e66665. https://doi.org/10.1371/journal.pone.0066665
Prado SG, Collazo JA, Marand MH, Irwin RE (2021) The influence of floral resources and microclimate on pollinator visitation in an agro-ecosystem. Agric Ecosyst Environ 307:107196–107204. https://doi.org/10.1016/j.agee.2020.107196
Rahman PM, Varma RV, Sileshi GW (2012) Abundance and diversity of soil invertebrates in annual crops, agroforestry and forest ecosystems in the Nilgiri biosphere reserve of Western Ghats, India. Agroforest Syst 85:165–177. https://doi.org/10.1007/s10457-011-9386-3
Rainio J, Niemela J (2003) Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers Conserv 12:487–506. https://doi.org/10.1023/A:1022412617568
Romeis J, Romeis 0, Shanower TG (1995) Paratrechina longicornis (Hymenoptera: Formicidae), a predator of Helicoverpa armigera (Lepidoptera: Noctuidae) eggs. Biol Control 9:56–58
Santos-Heredia C, Andresen E, Zàtare DA, Escobar F (2018) Dung beetles and their ecological functions in three agroforestry systems in the Lacandona rainforest of Mexico. Biodivers Conserv 27:2379–2394. https://doi.org/10.1007/s10531-018-1542-x
Saputra DD, Sari RR, Hairiah K, Roshetko JM, Suprayogo D, van Noordwijk M (2020) Can cocoa agroforestry restore degraded soil structure following conversion from forest to agricultural use? Agroforest Syst 94:2261–2276. https://doi.org/10.1007/s10457-020-00548-9
Schroth G, Läderach P, Blackburn Cuero DS, Neilson J, Bunn C (2015) Winner or loser of climate change? A modelling study of current and future climatic suitability of Arabica coffee in Indonesia. Reg Environ Chang 15:1473–1482. https://doi.org/10.1007/s10113-014-0713-x
Silva RS, Tomaz AC, Lopes MC, Martins JC, Xavier VM, Picanço MC (2015) Toxicity of botanical insecticides on Diaphania hyalinata, their selectivity for the predatory ant Paratrechina sp., and their potential phytotoxicity on pumpkin. Int J Pest Manage 62:1–10. https://doi.org/10.1080/09670874.2015.1111466
Smith C, Barton D, Johnson MD, Wendt C, Milligan MC, Njoroge P, Gichuki P (2015) Bird communities in sun and shade coffee farms in Kenya. Glob Ecol Conserv 4:479–490. https://doi.org/10.1016/j.gecco.2015.09.004
Spooner FEB, Pearson RG, Freeman R (2018) Rapid warming is associated with population decline among terrestrial birds and mammals globally. Glob Change Biol 24:4521–4531. https://doi.org/10.1111/gcb.14361
Staley JT, Hodgson CJ, Mortimer SR, Morecroft MD, Masters GJ, Brown VK, Taylor ME (2007) Effects of summer rainfall manipulations on the abundance and vertical distribution of herbivorous soil macro-invertebrates. Eur J Soil Biol 43:189–198. https://doi.org/10.1016/j.ejsobi.2007.02.010
Swengel AB (2001) A literature review of insect responses to fire, compared to other conservation managements of open habitat. Biodivers Conserv 10:1141–1169. https://doi.org/10.1023/A:1016683807033
Tscharntke T, Clough Y, Bhagwat SA et al (2011) Multifunctional shade-tree management in tropical agroforestry landscapes—a review. J Appl Ecol 48:619–629. https://doi.org/10.1111/j.1365-2664.2010.01939.x
Van Klink R, Bowler DE, Gongalsky KB, Swengel AB, Gentile A, Chase JM (2020) Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368:417–420
von Rintelen K, Arida E, Häuser C (2017) A review of biodiversity-related issues and challenges in megadiverse Indonesia and other Southeast Asian countries. RIO 3:e20860. https://doi.org/10.3897/rio.3.e20860
Woodcock BA (2005) Pitfall trapping in ecological studies. In: Leather S (ed) Insect sampling in forest ecosystems. Blackwell Publishing, Oxford, pp 37–57
Prajanti SDW, Pramono SE, Adzim F (2020) Factors influencing Indonesia coffee exports volume. In: International conference on research and academic community services (ICRACOS 2019). https://doi.org/10.2991/icracos-19.2020.8
Szenthe A (2020) Top coffee producing countries. WorldAtlas. Accessed 23 May 2021
Wei T, Simko V (2021) R package “corrplot”. Visualization of a correlation matrix
Acknowledgements
We thank Indonesia RISTEK and the regional Perhutani and BKSDA for authorising the study. We thank our field team E. Adinda, D. Ahmad, N. Ahmad, M. Balestri, H. Birot, K. Hedger, R. Hidayat, G. Himawan, S. Manson, Y. Nazmi, A. Nunur, D. Rustandi. We thank two anonymous reviewers for constructive feedback and suggestions for improvement.
Funding
Augsburg Zoo, Brevard Zoo, Cleveland Zoo and Zoo Society, Columbus Zoo and Aquarium, Disney Worldwide Conservation Fund, Global Challenges Fund, Henry Doorly Zoo, International Primate Protection League, Little Fireface Project, Mohamed bin al Zayed Species Conservation Fund (152511813, 182519928), Margot Marsh Biodiversity Fund, Memphis Zoo, Moody Gardens Zoo, Paradise Wildlife Park, People’s Trust for Endangered Species, Phoenix Zoo, Primate Action Fund, Shaldon Wildlife Trust, Sophie Danforth Conservation Biology Fund, and ZGAP provided the funding for this project.
Author information
Authors and Affiliations
Contributions
Conceptualization: MC, TB, KAIN; Methodology: MC, TB, KAIN; Formal analysis and investigation: MC, TB; BHF; Writing—original draft preparation: MC; Writing—review and editing: all authors; Funding acquisition: MC, KAIN; Resources: BB; MAI; KAIN; Supervision: BB; MAI; KAIN; Visualization: MC; Software: MC; Data curation: MC, TB, BHF, KAIN; Investigation: MC, TB, BHF; Project administration: MC, KAIN.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Campera, M., Budiadi, B., Bušina, T. et al. Abundance and richness of invertebrates in shade-grown versus sun-exposed coffee home gardens in Indonesia. Agroforest Syst 96, 829–841 (2022). https://doi.org/10.1007/s10457-022-00744-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-022-00744-9