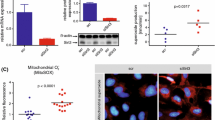
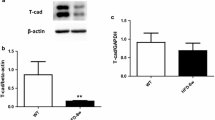
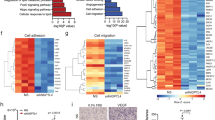
Abstract
Introduction
Although thioredoxin-interacting protein (TXNIP) is involved in a variety of biological functions, the contribution of endothelial TXNIP has not been well-defined in regards to endothelial and vascular function or in post-ischemic revascularisation. We postulated that inhibition of endothelial TXNIP with siRNA or in a Cre-LoxP system could be involved in protection from high fat, high protein, low carbohydrate (HFHPLC) diet-induced oxidative stress and endothelial dysfunction, leading to vascular damage and impaired revascularisation in vivo.
Methods and results
To investigate the role of endothelial TXNIP, the TXNIP gene was deleted in endothelial cells using anti-TXNIP siRNA treatment or the Cre-LoxP system. Murine models were fed a HFHPLC diet, known to induce metabolic disorders. Endothelial TXNIP targeting resulted in protection against metabolic disorder-related endothelial oxidative stress and endothelial dysfunction. This protective effect mitigates media cell loss induced by metabolic disorders and hampered metabolic disorder-related vascular dysfunction assessed by aortic reactivity and distensibility. In aortic ring cultures, metabolic disorders impaired vessel sprouting and this alteration was alleviated by deletion of endothelial TXNIP. When subjected to ischemia, mice fed a HFHPLC diet exhibited defective post-ischemic angiogenesis and impaired blood flow recovery in hind limb ischemia. However, reducing endothelial TXNIP rescued metabolic disorder-related impairment of ischemia-induced revascularisation.
Conclusion
Collectively, these results show that targeting endothelial TXNIP in metabolic disorders is essential to maintaining endothelial function, vascular function and improving ischemia-induced revascularisation, making TXNIP a potential therapeutic target for therapy of vascular complications related to metabolic disorders.





Similar content being viewed by others
References
Clifton P (2009) High protein diets and weight control. Nutr Metab Cardiovasc Dis 19:379–382. https://doi.org/10.1016/j.numecd.2009.02.011
Bedarida T, Baron S, Vessieres E et al (2014) High-protein-low-carbohydrate diet: deleterious metabolic and cardiovascular effects depend on age. Am J Physiol Heart Circ Physiol 307:H649–H657. https://doi.org/10.1152/ajpheart.00291.2014
Burcelin R, Crivelli V, Dacosta A et al (2002) Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 282:E834–E842. https://doi.org/10.1152/ajpendo.00332.2001
Foo SY, Heller ER, Wykrzykowska J et al (2009) Vascular effects of a low-carbohydrate high-protein diet. Proc Natl Acad Sci USA 106:15418–15423. https://doi.org/10.1073/pnas.0907995106
Garg PK, Biggs ML, Carnethon M et al (2014) Metabolic syndrome and risk of incident peripheral artery disease. Hypertension 63:413–419. https://doi.org/10.1161/HYPERTENSIONAHA.113.01925
Richter L, Freisinger E, Lüders F et al (2018) Impact of diabetes type on treatment and outcome of patients with peripheral artery disease. Diabetes Vasc Dis Res 15:504–510. https://doi.org/10.1177/1479164118793986
Incalza MA, D'Oria R, Natalicchio A et al (2018) Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc Pharmacol 100:1–19. https://doi.org/10.1016/j.vph.2017.05.005
Jia G, Sowers JR (2014) Endothelial dysfunction potentially interacts with impaired glucose metabolism to increase cardiovascular risk. Hypertension 64:1192–1193. https://doi.org/10.1161/HYPERTENSIONAHA.114.04348
Dawber TR, Meadors GF, Moore FE (1951) Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 41:279–281. https://doi.org/10.2105/ajph.41.3.279
Ungvari Z, Tarantini S, Kiss T et al (2018) Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol 15:555–565. https://doi.org/10.1038/s41569-018-0030-z
Albadawi H, Oklu R, Cormier NR et al (2014) Hind limb ischemia-reperfusion injury in diet-induced obese mice. J Surg Res 190:683–691. https://doi.org/10.1016/j.jss.2014.01.020
Couffinhal T, Silver M, Kearney M et al (1999) Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 99:3188–3198. https://doi.org/10.1161/01.cir.99.24.3188
Bir SC, Pattillo CB, Pardue S et al (2014) Nitrite anion therapy protects against chronic ischemic tissue injury in db/db diabetic mice in a NO/VEGF-dependent manner. Diabetes 63:270–281. https://doi.org/10.2337/db13-0890
Silvestre J-S, Smadja DM, Lévy BI (2013) Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev 93:1743–1802. https://doi.org/10.1152/physrev.00006.2013
Cai H, Harrison DG (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87:840–844. https://doi.org/10.1161/01.res.87.10.840
Collins AR, Lyon CJ, Xia X et al (2009) Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res 104:e42–e54. https://doi.org/10.1161/CIRCRESAHA.108.188771
Csiszar A, Ungvari Z, Edwards JG et al (2002) Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90:1159–1166. https://doi.org/10.1161/01.res.0000020401.61826.ea
Nivet-Antoine V, Labat C, Shamieh El S et al (2013) Relationship between catalase haplotype and arterial aging. Atherosclerosis 227:100–105. https://doi.org/10.1016/j.atherosclerosis.2012.12.015
Ebrahimian TG, Heymes C, You D et al (2006) NADPH oxidase-derived overproduction of reactive oxygen species impairs postischemic neovascularization in mice with type 1 diabetes. Am J Pathol 169:719–728. https://doi.org/10.2353/ajpath.2006.060042
Haddad P, Dussault S, Groleau J et al (2011) Nox2-derived reactive oxygen species contribute to hypercholesterolemia-induced inhibition of neovascularization: effects on endothelial progenitor cells and mature endothelial cells. Atherosclerosis 217:340–349. https://doi.org/10.1016/j.atherosclerosis.2011.03.038
Warren CM, Ziyad S, Briot A et al (2014) A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci Signal 7:ra1. https://doi.org/10.1126/scisignal.2004235
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948. https://doi.org/10.1038/nature04634
Hebert-Schuster M, Fabre EE, Nivet-Antoine V (2012) Catalase polymorphisms and metabolic diseases. Curr Opin Clin Nutr Metab Care 15:397–402. https://doi.org/10.1097/MCO.0b013e328354a326
Junn E, Han SH, Im JY et al (2000) Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 164:6287–6295. https://doi.org/10.4049/jimmunol.164.12.6287
Kim GS, Jung JE, Narasimhan P et al (2012) Induction of thioredoxin-interacting protein is mediated by oxidative stress, calcium, and glucose after brain injury in mice. Neurobiol Dis 46:440–449. https://doi.org/10.1016/j.nbd.2012.02.008
Bedarida T, Baron S, Vibert F et al (2016) Resveratrol decreases TXNIP mRNA and protein nuclear expressions with an arterial function improvement in old mice. J Gerontol A 71:720–729. https://doi.org/10.1093/gerona/glv071
Shah A, Xia L, Goldberg H et al (2013) Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 288:6835–6848. https://doi.org/10.1074/jbc.M112.419101
Schulze PC, Yoshioka J, Takahashi T et al (2004) Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem 279:30369–30374. https://doi.org/10.1074/jbc.M400549200
Nivet-Antoine V, Cottart C-H, Lemaréchal H et al (2010) Trans-resveratrol downregulates Txnip overexpression occurring during liver ischemia-reperfusion. Biochimie 92:1766–1771. https://doi.org/10.1016/j.biochi.2010.07.018
Oberacker T, Bajorat J, Ziola S et al (2018) Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS Lett 592:2297–2307. https://doi.org/10.1002/1873-3468.13156
Bedarida T, Domingues A, Baron S et al (2018) Reduced endothelial thioredoxin-interacting protein protects arteries from damage induced by metabolic stress in vivo. FASEB J 32:3108–3118. https://doi.org/10.1096/fj.201700856RRR
Zhou R, Tardivel A, Thorens B et al (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nature Immunol 11:136–140. https://doi.org/10.1038/ni.1831
Zhou J, Yu Q, Chng W-J (2011) TXNIP (VDUP-1, TBP-2): a major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int J Biochem Cell Biol 43:1668–1673. https://doi.org/10.1016/j.biocel.2011.09.005
Parikh H, Carlsson E, Chutkow WA et al (2007) TXNIP regulates peripheral glucose metabolism in humans. PLoS Med 4:e158. https://doi.org/10.1371/journal.pmed.0040158
Dunn LL, Simpson PJL, Prosser HC et al (2014) A critical role for thioredoxin-interacting protein in diabetes-related impairment of angiogenesis. Diabetes 63:675–687. https://doi.org/10.2337/db13-0417
Elshaer SL, Mohamed IN, Coucha M et al (2017) Deletion of TXNIP mitigates high-fat diet-impaired angiogenesis and prevents inflammation in a mouse model of critical limb ischemia. Antioxidants 6:E47. https://doi.org/10.3390/antiox6030047
Jeong M, Piao Z-H, Kim MS et al (2009) Thioredoxin-interacting protein regulates hematopoietic stem cell quiescence and mobilization under stress conditions. J Immunol 183:2495–2505. https://doi.org/10.4049/jimmunol.0804221
Arruda DC, Schlegel A, Bigey P, Escriou V (2016) Lipoplexes strengthened by anionic polymers: easy preparation of highly effective siRNA vectors based on cationic lipids and anionic polymers. Methods Mol Biol 1445:137–148. https://doi.org/10.1007/978-1-4939-3718-9_8
Alva JA, Zovein AC, Monvoisin A et al (2006) VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn 235:759–767. https://doi.org/10.1002/dvdy.20643
Yoshioka J, Chutkow WA, Lee S et al (2012) Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Investig 122:267–279. https://doi.org/10.1172/JCI44927
Shireman PK, Quinones MP (2005) Differential necrosis despite similar perfusion in mouse strains after ischemia. J Surg Res 129:242–250. https://doi.org/10.1016/j.jss.2005.06.013
Nebuloni L, Kuhn GA, Müller R (2013) A comparative analysis of water-soluble and blood-pool contrast agents for in vivo vascular imaging with micro-CT. Acad Radiol 20:1247–1255. https://doi.org/10.1016/j.acra.2013.06.003
Baker M, Robinson SD, Lechertier T et al (2011) Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc 7:89–104. https://doi.org/10.1038/nprot.2011.435
Cha-Molstad H, Saxena G, Chen J, Shalev A (2009) Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J Biol Chem 284:16898–16905. https://doi.org/10.1074/jbc.M109.010504
Baron S, Bedarida T, Cottart C-H et al (2014) Dual effects of resveratrol on arterial damage induced by insulin resistance in aged mice. J Gerontol A 69:260–269. https://doi.org/10.1093/gerona/glt081
Sun J, Deng H, Zhou Z et al (2018) Endothelium as a potential target for treatment of abdominal aortic aneurysm. Oxid Med Cell Longev 2018:6306542–6306612. https://doi.org/10.1155/2018/6306542
Wu D, Ren P, Zheng Y et al (2017) NLRP3 (nucleotide oligomerization domain–like receptor family, pyrin domain containing 3)–caspase-1 inflammasome degrades contractile proteins. Arterioscler Thromb Vasc Biol 37:694–706. https://doi.org/10.1161/ATVBAHA.116.307648
Gao L, Siu KL, Chalupsky K et al (2012) Role of uncoupled endothelial nitric oxide synthase in abdominal aortic aneurysm formation. Hypertension 59:158–166. https://doi.org/10.1161/HYPERTENSIONAHA.111.181644
Siu KL, Li Q, Zhang Y et al (2017) NOX isoforms in the development of abdominal aortic aneurysm. Redox Biol 11:118–125. https://doi.org/10.1016/j.redox.2016.11.002
Moon SK, Thompson LJ, Madamanchi N et al (2001) Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 280:H2779–H2788. https://doi.org/10.1152/ajpheart.2001.280.6.H2779
Gaubert ML, Sigaudo-Roussel D, Tartas M et al (2007) Endothelium-derived hyperpolarizing factor as an in vivo back-up mechanism in the cutaneous microcirculation in old mice. J Physiol 585:617–626. https://doi.org/10.1113/jphysiol.2007.143750
Gendron M-È, Thorin-Trescases N, Villeneuve L, Thorin E (2007) Aging associated with mild dyslipidemia reveals that COX-2 preserves dilation despite endothelial dysfunction. Am J Physiol Heart Circ Physiol 292:H451–H458. https://doi.org/10.1152/ajpheart.00551.2006
Forrester MT, Seth D, Hausladen A et al (2009) Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J Biol Chem 284:36160–36166. https://doi.org/10.1074/jbc.M109.057729
Hattori K, Heissig B, Wu Y et al (2002) Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med 8:841–849. https://doi.org/10.1038/nm740
Takahashi T, Kalka C, Masuda H et al (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438
Nowak-Sliwinska P, Alitalo K, Allen E et al (2018) Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 21:425–532. https://doi.org/10.1007/s10456-018-9613-x
Farrell MR, Rogers LK, Liu Y et al (2010) Thioredoxin-interacting protein inhibits hypoxia-inducible factor transcriptional activity. Free Radic Biol Med 49:1361–1367. https://doi.org/10.1016/j.freeradbiomed.2010.07.016
Shin D, Jeon JH, Jeong M et al (2008) VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim Biophys Acta 1783:838–848. https://doi.org/10.1016/j.bbamcr.2007.10.012
Simons M, Alitalo K, Annex BH et al (2015) State-of-the-art methods for evaluation of angiogenesis and tissue vascularization: a scientific statement from the American Heart Association. Circ Res 116:e99–e132. https://doi.org/10.1161/RES.0000000000000054
Das NM, Hatsell S, Nannuru K et al (2016) In vivo quantitative microcomputed tomographic analysis of vasculature and organs in a normal and diseased mouse model. PLoS ONE 11:e0150085–e0150118. https://doi.org/10.1371/journal.pone.0150085
Joseph C, Quach JM, Walkley CR et al (2013) Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell 13:520–533. https://doi.org/10.1016/j.stem.2013.10.010
Kogata N, Arai Y, Pearson JT et al (2006) Cardiac ischemia activates vascular endothelial cadherin promoter in both preexisting vascular cells and bone marrow cells involved in neovascularization. Circ Res 98:897–904. https://doi.org/10.1161/01.RES.0000218193.51136.ad
Kilani B, Gourdou Latyszenok V, Guy A et al (2019) Comparison of endothelial promoter efficiency and specificity in mice reveals a subset of Pdgfb-positive hematopoietic cells. J Thromb Haemost 17:827–840. https://doi.org/10.1111/jth.14417
Guerin CL, Loyer X, Vilar J et al (2015) Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: evidence of vasculogenic potential. Thromb Haemost 113:1084–1094. https://doi.org/10.1160/TH14-09-0748
Smadja DM (2017) Bone marrow very small embryonic-like stem cells: new generation of autologous cell therapy soon ready for prime time? Stem Cell Rev Rep 13:198–201. https://doi.org/10.1007/s12015-017-9718-4
Rossi E, Poirault-Chassac S, Bieche I et al (2019) Human endothelial colony forming cells express intracellular CD133 that modulates their vasculogenic properties. Stem Cell Rev Rep 15:590–600. https://doi.org/10.1007/s12015-019-09881-8
Acknowledgements
The authors thank all the technicians from the animal facilities of the Plateformes Mutualisées de l’Institut du Médicament (P-MIM-UMS3612 CNRS-US25 INSERM), Faculté de Pharmacie, Université de Paris; the Small Animals Platform of the Cochin Institute for Echo-Doppler assessment and Life Imaging, Université de Paris (Plateforme Imageries du Vivant-PIV-UMRS1016 INSERM) for micro-CT assessment. The authors also thank Lofti Slimani (EA2496 and PIV, Paris, France) for his advice, and Anna Lokajczyk, Amayelle Rey, Astrid De La Roche and Teddy Leguillier (INSERM UMRS-1140, Paris, France) for their technical assistance. A.D. was supported by funds from the French Ministry of Research and Technology. This work was funded by grants from INSERM, the Université de Paris and FRM (DGE20111123012). The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10456_2019_9704_MOESM1_ESM.tiff
Figure S1 Targeting endothelial TXNIP protects endothelium-independent mesenteric artery reactivity induced by metabolic disorders. (A–D) Reactivity of mesenteric arteries from siSCR- or siTXNIP-treated mice fed the HFHPLC diet (n = 6‒9/group). Results are expressed as means ± SEM and were analysed by two-way ANOVA, followed by a Bonferroni post hoc test. *p ≤ 0.05 (A) Concentration-response curves of contraction in response to phenylephrine. (B) Concentration-response curves for acetylcholine. (C) Concentration-response curves for acetylcholine with L-NAME pre-treatment. (D) Concentration-response curves for acetylcholine with NO donor sodium nitroprusside (SNP) pre-treatment (TIFF 14094 kb)
Rights and permissions
About this article
Cite this article
Domingues, A., Boisson-Vidal, C., Marquet de Rouge, P. et al. Targeting endothelial thioredoxin-interacting protein (TXNIP) protects from metabolic disorder-related impairment of vascular function and post-ischemic revascularisation. Angiogenesis 23, 249–264 (2020). https://doi.org/10.1007/s10456-019-09704-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-019-09704-x