Abstract
Urban and agricultural expansion and intensification pose a critical threat to water quality and aquatic ecosystems. Increased nutrient loading into waterways combined with warming temperatures due to climate change have increased eutrophication and algal blooms. The relationship between land use, nutrient availability, and algal growth can vary dramatically across space and time, but few studies have captured this variation. The goal of this research is to assess water quality across time and disparate land uses, and its influence on algal community composition in the Albemarle Sound, a brackish water estuary in North Carolina. We collected water quality data from 21 sites across the sound, visiting six sites in Chowan County biweekly and 15 other sites twice between June and August 2020. Water samples from each site were tested for nitrate, phosphate, ammonia, bicarbonate, and total phosphorus (TP). Preserved algal samples from the six Chowan County sites were enumerated under a microscope to estimate genus richness and biomass. In the Chowan County sites, phosphorus increased and nitrate decreased over the course of the summer. Across all sites, TP increased with development and agricultural land use. These results suggest that sources of nitrogen and phosphorus in the sound differ. Algal richness increased with nitrate concentration and decreased with precipitation while biomass increased with water temperature. Our results indicate that climate change impacts, particularly increasing temperatures and extreme precipitation, influence how land use, water quality, and algal community composition interact. These data demonstrate the co-benefits of mitigating climate change in developing management strategies to reduce algal blooms.
Similar content being viewed by others
Introduction
Urban and agricultural expansion pose a critical threat to water quality and aquatic ecosystems. Due to increased nutrient loading from these land uses, harmful algal blooms (HABs) have become increasingly prevalent (Carpenter et al. 1998; Glibert et al. 2014; Paerl and Otten 2013). Patterns associated with climate change, namely warming temperatures and more intense and frequent precipitation, have also exacerbated harmful algal blooms by fostering ideal conditions for algal growth and increasing nutrient pollution into waterways during heavy precipitation (Paerl and Otten 2013). However, it is often unclear which nutrients and other water quality variables drive algal growth. Elucidating the underlying causes of these blooms is crucial to mitigating their impact on humans and natural ecosystems.
Land use types within a watershed can impact the quality of the water it drains. In the USA, most nitrogen (N) and phosphorus (P) runoff into surface water comes from nonpoint sources of pollution (Carpenter et al. 1998). Agriculture is a major nonpoint pollution source as fertilizers contain N and P compounds (Carpenter et al. 1998; Glibert et al. 2014; Huang et al. 2017; Le et al. 2010). Nutrients like inorganic N are often lost from agricultural fields through leaching or runoff into surface water, increasing nutrient levels downstream (Galloway et al. 2003, Glibert et al. 2014). Urban and suburban land can also influence nutrient runoff (Carpenter et al. 1998; Hobbie et al. 2017; Nielson and Smith 2005). For example, lawn fertilizers comprise a large portion of N loading in urban watersheds while pet waste is a major source of P (Hobbie et al. 2017). Impervious land area impacts nutrient loading by preventing stormwater from permeating into soil, therefore increasing runoff and nutrient leaching into waterways (Brush 2009; Liu et al. 2013; Xian et al. 2007).
Excess nutrient loading into water bodies can cause algal blooms (Fig. S1, Conley et al. 2009; Galloway et al. 2003; Glibert et al. 2005; Paerl and Otten 2013). Algal blooms pose a severe threat to aquatic ecosystems, with potential consequences for human health. Blooms can result in dead zones and fish kills (Moorman et al. 2014; Paerl and Otten 2013; Watson et al. 2016). Eutrophication and algal blooms may also increase water turbidity, which can negatively impact photosynthetic aquatic vegetation and estuarine predators, further limiting biodiversity (Lunt and Smee 2020; Paerl and Otten 2013, Sukenik et al. 2015). Additionally, some species of blue-green algae (cyanobacteria) secrete toxins like microcystins, a type of cyanotoxin which is connected to a range of health issues in humans, including rashes, liver failure, and cancer (Cheung et al. 2013; Lopez et al. 2008; Paerl 2008; Vranješ and Jovanović, 2011). Dinoflagellates, another type of plankton, also commonly produce toxins and form HABs (e.g., red tide; Fleming et al. 2011). To better protect aquatic ecosystems and human health from this threat, the causes and effects of algal blooms must be explored further.
It was historically assumed that algae in freshwater environments were limited by the availability of P (Schindler 1977) and brackish and coastal waters were predominantly N-limited. However, mounting evidence suggests that algae are more often co-limited by the availability of both N and P (Bratt et al. 2019; Elser et al. 2007; Harpole et al. 2011). Beyond evidence of co-limitation, within communities the availability of N and P can have varying effects on primary producer growth. Non-N-fixing genera thrive in water with higher N:P ratios compared to N-fixers (Berry et al. 2017; Paerl and Otten 2013). Furthermore, some species of algae can respond to changing nutrient availability in their environments. For example, Harke et al. (2016) found that P-scavenging gene expression in Microcystis, a non-N-fixer, increased when P concentration was low in Lake Erie, giving this genus an advantage over N-fixing algae when phosphate concentrations are low. Other species of microalgae in addition to Microcystis can uptake excess phosphate and store it in specialized cells (Powell et al. 2011). Thus, while nutrient availability influences algal growth, algae can impact nutrient concentrations through uptake and N-fixation. However, nutrient availability itself can vary dramatically across space and time due to factors including human activity, yet few studies have captured this spatiotemporal variation.
The goal of this research is to explore the relationship between space, time, land use, water quality, and algal community composition in the Albemarle Sound region of North Carolina. While much research on water quality and algal blooms has been conducted in the Neuse River Estuary (e.g., Cira et al. 2016; Paerl et al. 1998, 2004, 2010, Pamlico Sound (e.g., Bales 2003; Christian et al. 2004; Paerl et al. 2001, 2007; Piehler et al. 2004), and entire Albemarle-Pamlico Estuary (e.g., Lin et al. 2007; Tester et al. 2003), little research has focused specifically on the Albemarle Sound (e.g., Hazen and Esch 1983; Lombardo 2018; Moorman et al. 2014; Zhang et al. 2014). Thus, the Albemarle Sound is an understudied region despite its ecological importance as a habitat for many aquatic species (APNEP., n.d.-a, APNEP, n.d.-b).
This research seeks to answer the following questions: (1) How does land use influence water quality in the Albemarle Sound? (2) How does water quality vary across space and time? (3) How does water quality relate to algal taxa richness and biomass? Understanding the interaction between land use and water and how algal communities respond to these factors is crucial to determining potential management strategies and protecting an important estuary and the people who live near it.
Methods
Study area
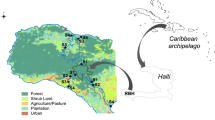
The Albemarle Sound (Fig. 1) is part of the Albemarle-Pamlico estuarine system. Contained by barrier islands, it is the second largest estuary in the USA. (APNEP, n.d.-c). The estuary is composed of a diverse assemblage of aquatic biota, such as blue crabs, shrimp, oysters, and several fish species, including Atlantic Sturgeon which rely on the estuary to breed (APNEP, n.d.-a; APNEP, n.d.-b). The sound also provides economic services to northeastern North Carolina including commercial fishing and aquaculture value as well as recreational value (Van Houtven et al. 2016). The Chowan, Pasquotank, and Roanoke river basins, which extend into Virginia, feed into the sound (APNEP, n.d.-c). The region is threatened by climate change and anthropogenic activity. The rate of relative sea level rise has increased, with potential negative impacts on the estuary ecosystem (Bhattachan et al. 2018; Kemp et al. 2009). Furthermore, agriculture and development in the region have contributed to eutrophication, erosion, and loss of wetlands (APNEP, n.d.-c). The region regularly experiences algal blooms in the summer months (Plaas et al. 2022).
Map of study region. Sampling was conducted at 21 sites across 8 counties in Northeastern North Carolina. Sites in Chowan County (1–6) were sampled every two weeks from June 8th to August 10th for a total of five times while the remaining sites (7–21) were each sampled twice across June and July. The watersheds for each site are outlined in green. We obtained state outlines from the USA Census Bureau (2018) and county outlines from the NCDEQ (2019)
Field sampling
Field sampling took place over 10 weeks in Chowan County, NC, and 7 other nearby counties (Fig. 1) during summer 2020. Sampling locations were based on public access points and selected to include multiple land use types (e.g., agricultural, developed, wetland) and different locations along an upstream–downstream gradient. Each site in Chowan County (n = 6) was monitored biweekly (every two weeks), while all other sites (n = 15) were monitored once a month (a total of 2 times) to capture both spatial and temporal variation. Secchi depth was measured as a proxy for turbidity and a probe was used to determine dissolved oxygen concentration (Hanna Instruments, HI9142). pH, total dissolved solids (TDS), conductivity (as a proxy for salinity), and temperature were measured on site using a Hanna Instruments Combo (HI98129). Water samples were collected at 0.25 m depth. Weather data were collected from the North Carolina Climate Retrieval and Observations Network of the Southeast Database (North Carolina Climate Office 2020).
Laboratory analysis
Water samples were filtered using 0.45-μm nylon syringe filters and colorimetrically analyzed for nitrate (NO3−, cadmium reduction method), phosphate (PO4, ascorbic acid reduction method), and ammonia (NH3, nesslerization method) using a LaMotte Smart 3 colorimeter. Bicarbonate (HCO3−) concentration was measured using a LaMotte titration kit (Hardness Titration Reagent). Phosphate was analyzed using a vanadomolybdophosphoric acid method initially for 10 of the study sites. This method was unable to detect phosphate levels, so those measurements were not included in the analysis and an analysis suitable to lower concentrations (ascorbic acid reduction) was used for the remaining samples. Unfiltered water samples from all sites (excluding the first samples from 10 study sites) were frozen and later tested for total phosphorus (TP, ascorbic acid reduction with persulfate digestion method) using a LaMotte Smart 3 Colorimeter.
Algal sample enumeration
Algal samples from the 6 Chowan County sites were preserved with Lugol’s solution (with the concentration dependent on the abundance of algae in the sample) and frozen for six to seven months. Samples were then enumerated using a microscope (Martin Microscope Co. 24,321). One millilter was drawn from each sample and placed in a centrifuge for 1 min at 100% speed (15,000 rpm). The supernatant was poured off and the sample was resuspended with 0.5–2 mL of distilled water and 0.1 mL of sample was analyzed on a Palmer cell. Cell counts were conducted by examining 100 fields of view, except in the case of one sample where 50 fields of view were analyzed. A Whipple grid was used to aid in genus identification by determining cell size. There were five unknown groups categorizing cells whose genera remained unidentified. These were included in genus richness and biovolume. All cell counts were later standardized to 100 fields of view and 1 mL of original sample.
Genus richness was calculated as the total number of genera identified in each sample. The biovolume of each genus was calculated by multiplying the standardized counts by an average genus biovolume. Following Passy and Legendre (2006), biovolume was used as a proxy for algal biomass. Biovolume was determined using data from the Swedish Meteorological and Hydrological Institute’s Nordic Microalgae and Aquatic Protozoa database, phycology data from the Academy of Natural Sciences and Patrick Center for Environmental Research at Drexel University (ANSP, 2001), a diatom biovolume dataset compiled by Leblanc et al. (2012), and other resources (García et al. 2007; Harrison et al. 2015; Hawkins et al. 2005; Olenina et al. 2006; SMHI, n.d.; Università del Salento, n.d.). Because datasets included measurements for several species of many genera, only measurements which were plausible for the samples collected in this study were included in the average biovolume calculation. Biovolumes of unknown groups were calculated based on Hillebrand et al. (1999).
Statistical analysis
Watersheds were delineated using StreamStats (USGS, n.d.). The influence of land use on each sampling point was determined by clipping the National Land Cover Database (NLCD) (2016) land cover layer (USGS 2016) to each watershed in ArcGIS Pro 2.5.0 (ESRI 2020) and calculating the proportion of each land use: open water, developed (open space, low, medium, and high intensity), barren land, forest (deciduous, evergreen, mixed), shrub/scrub, herbaceous, agriculture (pasture and cropland), and wetland (woody and emergent herbaceous). ArcGIS Pro was further used to calculate site distance to river mouth.
Linear modeling was used to assess how nutrient concentrations in the Chowan County sites varied over the course of the summer. The models that best fit the data for each water quality variable, NO3−, PO4, NH3, HCO3−, and TP, were determined using stepwise AIC model selection (Table S1). Proportion developed, agricultural, forested, and wetland area in the watershed, antecedent precipitation, date, distance to river mouth (km), and site were included in the modeling. Antecedent precipitation was determined by averaging the daily precipitation (mm) from the week prior to when the sample was collected.
Linear modeling was further used to assess relationships of land use to nutrient concentration among all sites. Similar to the temporal analysis of nutrient concentrations at the Chowan sites, models were chosen using stepwise AIC model selection, taking into account proportion developed, agricultural, forested, wetland area, and distance to river mouth (km; Table S2). Nutrient concentration data were averaged for each site. Because not all sites were visited consistently across time, date and precipitation were excluded from this analysis. Site was also removed as the 21 separate sites created too many variables for the model to run. All statistical analyses were conducted in RStudio version 3.6.2 (RStudio Team 2019).
Linear modeling was also used to determine the relationship between nutrient concentration and algal genus richness, biomass, and percent total biomass of cyanobacteria, diatoms, and dinoflagellates (Table S3, Table S4, Table S5). For genus richness and biomass, AIC model selection was used to find the best model among the following variables: NO3−, PO4, NH3, and HCO3− concentration, precipitation, site name, date, proportion forest, agriculture, wetland, and developed land, distance from river mouth, water temperature, Secchi depth, pH, conductivity, and TDS. Total phosphorus could not be included in model selection as TP was not collected for the first two samples (sites 5 and 6; June 13). Percent algal biomass was calculated for each genus in each sample to discern any differences in which algae types were dominant between sites. To assess the influence of nutrient concentrations, and water temperature and salinity on the proportion total biomass of cyanobacteria, diatoms, and dinoflagellates, stepwise AIC model selection for the percent biomass of those algal groups included NO3−, PO4, NH3, and HCO3− concentrations, water temperature, site, and conductivity.
Results
Watersheds varied in size and land use, with development, agriculture, forest, and wetland-dominated watersheds. Agriculture was the most predominant land use in study sites’ watersheds, followed by wetland, developed land, and forest (Table 1). Agriculture composed the greatest proportion of land area in 11 sites, while wetland, forest, and developed land made up the greatest proportion of land area in 4, 3, and 3 sites, respectively. Algal blooms were observed at some sites during the study period, two of which had watersheds dominated by agricultural land use while another had a predominantly forested watershed (Fig. S2).
Temporal variation in water quality
The linear model results for nutrient levels at the six Chowan County sites visited biweekly demonstrate that nitrate and phosphorus levels both changed over time, but in different ways (Table 2). NO3− decreased significantly over time (p < 0.05) while TP (p < 0.001) and PO4 (p < 0.05) increased over the course of the summer. There was also a significant, positive association between TP and precipitation (p < 0.001). While PO4 concentrations also increased with precipitation, this relationship was not significant (p > 0.05). Time did not influence NH3 concentration. HCO3− similarly did not vary significantly over time. Both NH3 and HCO3− concentrations were predominantly influenced by differences between sites (Fig. S3).
While the model chosen through stepwise AIC model selection did not include precipitation as a variable influencing NO3−, the general negative trend in nitrate concentration corresponded with a spike in rainfall at the end of the summer during Hurricane Isaias (Fig. 2). While TP concentrations were highest at most sites on the last day of sample collection, the NO3− levels at most sites were lower than previously observed (Fig. 2).
NO3− (left) and TP (right) concentrations and average weekly precipitation. The figures illustrate the NO3− and TP concentrations at each site and precipitation over the summer. Bars represent average weekly rainfall, patterned lines indicate different sites (n = 6), and points represent visits to each site (n = 30; n = 24). During the last week, Hurricane Isaias hit the region, causing a dramatic rise in precipitation from the previous week
Spatial variation in water quality
The linear modeling results for average nutrient concentrations across all study sites revealed that TP was the only nutrient measured that was significantly impacted by land use (Table 3). TP increased with increasing proportions of developed (p < 0.05) and agricultural (p < 0.05) land use in the watershed (Fig. 3). Both PO4 (p < 0.05) and NH3 (p < 0.05) exhibited significant, positive relationships with distance to river mouth, meaning sites farther downstream tended to have lower PO4 and NH3 levels (Fig. S4). HCO3− was negatively associated with proportion of developed land, farmland, forest, and wetland, as well as distance to river mouth, but these variables were not statistically significant (Table 3). The best fit model for NO3− did not include any explanatory variables (Table 3).
Relationship between proportion of agricultural land and TP. Points represent study sites (n = 21), best fit line shows the relationship between TP and proportion farmland, and shading indicates a 95% confidence interval. There was a significant, positive association between TP and proportion agricultural land cover in site watersheds among all study sites (p < 0.05). See Table 3 for slope based on full model
Variation in algal community composition
A total of 65 algal genera (12 Cyanobacteria, 16 Chlorophyta, 1 Charophyta, 3 Euglenozoa, 23 Bacillariophyceae (diatoms), and 10 Dinoflagellata) plus 5 unknown groups were identified in 26 samples from the six Chowan County sites (Table S6). Overall, the most abundant genera based on standardized cell counts (rounded to nearest whole number) were Microcystis (n = 9250), Nodularia (n = 2077), and Anabaena (n = 1402), while the genera with the greatest total biomass were Microcystis (3.2 × 105 μm3), Gymnodinium (2.4 × 105 μm3), and Nodularia (2.6 × 105 μm3; Table S6). Twenty-three (35.4%) of the identified genera have confirmed or potential toxic strains, of which 11 (47.8%) were Cyanobacteria and nine (39.1%) were dinoflagellates (Table S6). Six (9.2%) genera can fix atmospheric nitrogen, all Cyanobacteria (Table S6).
Algal genus richness ranged from seven to 38 genera. The sites with the greatest mean richness were sites 3 and 4, while sites 5 and 6 had the lowest average genus richness, but the greatest average biomass (Table S7). Sites 1 and 2 had the lowest average biomass (Table S7). Total standardized biomass ranged from a minimum of 1,015.05 μm3 to a maximum of 420,529.58 μm3 in one sample. However, differences in genus richness and total biomass between sites were not statistically significant (Fig. S5).
According to linear modeling results, date appeared to have the strongest influence on genus richness, with richness increasing over time (p < 0.001; Table 4). This relationship between algal richness and time held true for each site (Fig. 4). Genus richness was positively related to NO3− (p < 0.05) and HCO3− concentration (p < 0.01; Table 4). Richness was negatively associated with precipitation (p < 0.05) but increased with pH (p < 0.01; Table 4).
Relationship between algal genus richness and time by study site. Points represent visits to each site (n = 26); color-coded best fit lines represent different sites (n = 6). The linear modeling results indicate that algal genus richness increased significantly over time (p < 0.001), though site was not included in the best model
The best linear model for algal biomass included HCO3− concentration, water temperature, conductivity, and TDS (Table 5). Of these variables, biomass was significantly positively associated with water temperature (p < 0.001; Table 5; Fig. 5).
Relationship between total biomass (as biovolume) and water temperature. Points represent visits to each site (n = 26); best fit line represents the relationship between algal biomass and water temperature, and gray shading represents a 95% confidence interval. Algal biomass (measured as biovolume) increased significantly with water temperature (p < 0.001). See Table 5 for slope based on full model
Algal biomass composition (based on percent of total biomass) varied significantly across space and time. Mean proportions of total biomass of cyanobacteria, green algae, diatoms, dinoflagellates, euglenozoan, and unknowns for each site showed that biomass at sites 1 and 2 was dominated by diatoms, mean total biomass at sites 3 and 4 was mainly composed of dinoflagellates, while cyanobacteria largely comprised the mean biomass of sites 5 and 6 (Fig. S6). While different algal groups appeared to dominate different sites, only the mean percent total biomass of cyanobacteria differed significantly among groups (p < 0.01, Fig. S7). Algal community composition also shifted over time. The proportion total biomass composed of diatoms at sites 1 and 2 increased over time, peaking in August, while the proportion of dinoflagellate biomass at sites 3 and 4 was greatest in early July (Fig. 6). The relative biomass of cyanobacteria was overwhelmingly dominant at sites 5 and 6 in late July, with a drastic decline in early August as the relative biomass of dinoflagellates increased (Fig. 6).
Proportion biomass of algal groups including unknowns over time at each site. Stacked bar plots show the proportion biomass (measured as biovolume) of the 5 algal groups identified in this study and unknowns. The first two algal samples at sites 5 and 6 (collected on June 13th) were omitted from this chart as algal taxa data was not collected for the remaining four sites until the following week
Because cyanobacteria, diatoms, and dinoflagellates were the most prevalent algal groups in terms of proportion of total biomass, linear modeling was used to determine the influence of water quality (nutrient concentration, conductivity, and water temperature) on the relative biomass of these three algal groups. The relative biomass of cyanobacteria increased significantly with increasing NO3− concentrations and water temperature while dinoflagellate relative biomass was significantly negatively associated with NO3− (Table 6; Fig. 7). The model selected for diatom relative biomass did not include any of the variables fed into the model.
Relationship between nitrate concentration and relative cyanobacteria (left) and dinoflagellate (right) biomass (measured as biovolume). Points represent visits to each site (n = 26); best fit lines represent the relationships between nitrate concentration and cyanobacteria and dinoflagellate relative biomass, and gray shading represents a 95% confidence interval. Relative cyanobacteria biomass increased significantly with NO3− concentration (p < 0.01) while percent biomass of dinoflagellates decreased (p < 0.05). See Table 6 for slopes based on full model
Discussion
Our results demonstrate that water quality characteristics varied differently across space, time, and land use, with nitrate concentration predominantly driven by time and phosphorus concentration driven by time, precipitation, and land use. Analysis of the Chowan County sites (those visited biweekly) revealed that TP increased significantly over time and with precipitation while NO3− decreased over time. Across all 21 sites, TP significantly increased with proportion of agricultural and developed land use. Algal genus richness increased over time and with NO3− concentration but decreased with precipitation. However, algal biomass was significantly positively related to water temperature. Similarly, percent total biomass of specific algal groups varied with NO3− concentration and temperature. We explore these results below.
Nitrate and total phosphorus over time
The contrasting trends in nitrate and TP over time suggest that the two nutrients are influenced by different factors, like different sources or mobility. Nitrate concentrations decreased over time in the Chowan County sites, especially after Hurricane Isaias, indicating a dilution effect. Similar dilution trends were found with lower pesticide and nutrient levels, including TN and nitrate–N, in North Carolina’s lower Cape Fear River watershed, where concentrated animal feeding operations (CAFOs) largely contribute to nutrient loading, after Hurricane Floyd (Mallin et al. 2002; Mallin and Corbett 2006). Mallin and others (2002) suggest these low concentrations were due to denitrification (the conversion of NO3− to atmospheric N2; Burgin et al. 2011) in the floodplain. High turbidity from sediment disturbed during a storm limits light penetration into water and photosynthesis (Blain and Shears 2019; Schmidt et al. 2019), which can lead to hypoxic conditions that promote denitrification. After Hurricane Isaias, the water in the sound was cloudy and brown (Fig. S8), indicating mobilization of sediments. Thus, the decline in nitrate concentration across study sites observed in this study could also be due to denitrification.
Unlike nitrate, TP increased over time, especially after the hurricane. While heavy precipitation may cause a dilution of environmental contaminants, it has also been found to increase pollutant levels in waterways depending on surrounding land use. Some sites in North Carolina demonstrated above-average TN, TP, and ammonia levels after Hurricanes Fran, Bonnie, and Floyd. These nutrients could largely be traced to concentrated animal feeding operations (CAFOs) and human sewage (Mallin and Corbett 2006). The lack of dilution effect suggests that greater rainfall caused mobilization of a major source of phosphorus on land. Erosion or resuspension of sediment is a potential explanation for this observed increase in TP (Carpenter et al. 1998; Cordell et al. 2009; Peñuelas et al. 2013; Ulén and Kalisky 2005). Fertilizer application can influence soil P concentrations (Carpenter et al. 1998), so P levels may be higher in agricultural soils. In investigating the potential effects of various agricultural and climate change scenarios on N and P loading into the Baltic Sea, Huttunen et al. (2015) found that soil erosion and erosivity played a role in TP loading from agricultural soils. In addition to increasing turbidity facilitating denitrification, resuspension of sediments after a storm can release P from soils (House et al. 1998; Stutter et al. 2008). These results indicate that sources of phosphorus and nitrogen in the sound differ.
Land use and spatial variation
Nitrate was not significantly associated with the proportion of agricultural land use in a site’s watershed. In this region, agricultural land is overwhelmingly cultivated cropland (NLCD2016, n.d.). According to county profiles from the 2017 Census of Agriculture, the mean proportion of cropland in the eight counties included in the study area was 89% (predominantly soy and grains), compared to 1% pastureland and 8.25% woodland (Census of Agriculture—State and County Profiles—North Carolina 2017). The proportion of agricultural land cover in study site watersheds ranged between 0.24 and 85.79%. The lack of relationship between nitrate concentration and land use was surprising considering the prevalence of industrial agriculture in the region and variation in watershed land use (Savci 2012). According to the best fit model, none of the variables included in this study explained variation in nitrate concentrations. While agricultural fertilizers are known to contain nitrogenous compounds, the fertilizers used on farms in the study region are unknown. One fertilizer often used in the southeastern USA is a mix of urea and ammonium nitrate (called UAN; Environmental Defense Fund, n.d.). Determining which fertilizers are used on the farms surrounding the Albemarle Sound may help explain this result. Additionally, other factors may affect nitrate levels across the sound. Hobbie et al. (2017) found that atmospheric N deposition accounted for 34% of N inputs in seven urban watersheds, making it the second largest source of N. This atmospheric N comes from burning fossil fuels (Hobbie et al. 2017) and may be less relevant in more rural regions with lower population densities. However, there are other sources of nitrogen gases, like CAFOs and crop residue (Leytem et al. 2011; Tubiello et al. 2013). These potential sources of nitrate in the Albemarle region should be studied further.
Our results indicated that P increased with distance to river mouth (i.e., distance upstream). This finding contrasts with previous research that suggests higher phosphorus availability in coastal waters due to decreased phosphorus sequestration by iron (Blomqvist et al. 2004). For example, while Teichberg et al. (2010) found that P additions affected the growth of a macroalgal species in coastal waters, the researchers concluded that DIN was the main factor influencing growth, suggesting P was not limiting. In their meta-analysis, Elser et al. (2007) likewise observed that marine autotrophs are more limited by N than P, but that P-limitation may still occur in marine habitats. Because we do not know the TN concentrations at each site, it is difficult to determine the relative nutrient availability of these downstream sites, but the fact that ammonia followed the same pattern as phosphate suggests overall lower nutrient concentrations closer to the coast, potentially resulting in less algal growth than in upstream sites.
Climate change, water quality, and algae
Our results demonstrate that impacts associated with climate change, specifically increasing frequency and intensity of extreme weather events and rising water temperatures significantly influence algal community composition in the Albemarle Sound.
First, the negative relationship between richness and precipitation points to a dilution effect, similar to the apparent (but nonsignificant) influence of precipitation on NO3− concentration. Previous research in Danish lakes has demonstrated that precipitation can have a negative impact on diatom richness and lead to increased dominance of cyanobacteria (Özkan et al. 2013). This suggests that precipitation does not impact all algal taxa in the same way but can create conditions that favor the growth of certain taxa and lead to a decline in others, which would result in lower taxa richness.
The influence of precipitation on genus richness likely has implications for algal biomass. Decreased algal richness due to a dilution effect after heavy precipitation may create ideal conditions for one or few types of algae to dominate, leading to a bloom, especially if nutrient pollution from land enters the water as runoff (Özkan et al. 2013). While algal biomass in this study was not significantly influenced by precipitation, Lehman (2000) found that the biovolume of multiple algal taxa is greater with increased rainfall. Because the linear model only considered total algal biomass, changes in biomass of specific taxa in response to precipitation may not have been detected.
Total biomass was also not significantly related to nutrient concentration, yet N and P compounds are commonly known to stimulate algal growth (Altman and Paerl 2012; Bennett et al. 2001; Cordell et al. 2009; Galloway et al. 2003; Glibert et al. 2014; Savci 2012). The lack of relationship between biomass and nutrient concentration may be due to the inability to include TN and TP in the biovolume model. Only three N and P compounds were included in this analysis, so other nutrients may be influencing algal growth in this region. Additionally, TP was the only nutrient found to increase significantly with precipitation. Precipitation is a major pathway of nutrient loading into water, so TP is an important piece in analyzing this effect on algal biomass. Because extreme precipitation events are expected to increase in many regions including the southeastern USA due to climate change (Carter et al. 2018; Hayhoe et al. 2018), the influence of precipitation on algal community composition should be studied further.
Sites 5 and 6 had the lowest mean genus richness but the greatest mean biomass, demonstrating a potential inverse relationship between the two variables. Previous research has shown that phytoplankton richness is generally greatest at intermediate biovolumes (Passy and Legendre 2006; Spatharis et al. 2008). Passy and Legendre (2006) suggest that phytoplankton richness is lower at high biomasses in both nutrient-rich and nutrient-poor waters because phytoplankton grow in environments with relatively few ecological niches. With fewer niches to occupy, there is more competition between species as biomass increases.
Total algal biomass and relative biomass of cyanobacteria increased significantly with water temperature. This finding aligns with previous research which demonstrates that algae thrive in warmer water compared to some aquatic plants (Arnold et al. 2017; Beer et al. 1986; Paerl and Otten 2013). This relationship between water temperature and algae could threaten aquatic trophic interactions reliant upon submerged aquatic vegetation (SAV). While aquatic macrophytes can be more competitive in nutrient-poor environments (Barko and James 1998; van Donk and van de Bund 2002), Beer et al. (1986) found that the filamentous algae Lyngbya favors warmer temperatures, with an optimal temperature of 40 °C compared to 30 °C for the macrophyte Hydrilla. Increased nutrient loading into water bodies from human activities and higher water temperatures due to climate change may optimize algal growth (Paerl and Otten 2013) and result in a decline in aquatic macrophytes.
Our results also indicate that water quality influences algal taxa differently. For instance, the relative biomass of cyanobacteria increased significantly with nitrate concentration while relative dinoflagellate biomass was negatively associated with nitrate. While cyanobacterial blooms are sometimes caused by N-fixers and therefore are expected to respond more to P availability (Schindler 1977), the growth of non-N-fixers like Microcystis has been shown to increase with the addition of N compounds like nitrate and ammonium (Rückert and Giani 2004). While all N-fixing genera in this study were cyanobacteria, the non-N-fixing genus Microcystis largely dominated the group. Therefore, the relative biomass of nitrogen-fixing cyanobacteria did not greatly influence how the phylum responded to nutrient concentrations.
The inverse response of cyanobacteria and dinoflagellates to NO3− concentration may be because an increase in the proportion total biomass of cyanobacteria would cause a decrease in the proportion of dinoflagellate biomass and vice versa. Dinoflagellates and cyanobacteria seem to be particularly inversely related in sites 5 and 6, where cyanobacteria composed a large proportion of the total biomass in late July and were far less prevalent in early August when the relative biomass of dinoflagellates increased. This decline in relative cyanobacteria biomass during this time may be due to a dilution effect after Hurricane Isaias (Phlips et al. 2007), which similarly impacted nitrate concentration. Özkan et al. (2013) found the opposite effect of precipitation on cyanobacteria dominance in freshwater Danish lakes, but in our sites, saltwater intrusion following the hurricane may have created conditions favoring dinoflagellate genera, which most often thrive in marine ecosystems (de Vernal et al. 2013; White and Kaplan 2017). While conductivity was actually lower at sites 5 and 6 in August compared to other weeks, sampling was conducted several days after the hurricane, so these data may not have captured the impact of saltwater intrusion at those sites. More research in this region should examine the influence of conductivity and salinity on algal community composition.
Water quality and algal richness and biomass models were run excluding the last samples taken from each Chowan County site after Hurricane Isaias to illuminate the effects of the hurricane on water quality and better understand trends in non-hurricane conditions. TP concentration was significantly positively associated with proportion of agricultural land cover in the watershed, but not proportion of developed land cover (Table S9). The relationship between developed land and water quality may be stronger with the inclusion of post-hurricane data due to greater stormwater runoff into the sound during the hurricane. This change suggests that agricultural effects on water quality in this region are dominant until a major precipitation event. Additionally, algal richness was significantly negatively associated with conductivity (Table S10), while total algal biomass increased significantly with conductivity (Table S11). These relationships were not significant with post-hurricane data included. This difference may be because there was lower conductivity at each of the Chowan sites after the hurricane, which may have obscured these relationships. The positive association between biomass and conductivity may indicate a higher occurrence of algal blooms at sites closer to the coast or that high salinity gives few salt-tolerant taxa a competitive advantage over other algae. The specific taxa we analyzed (cyanobacteria, diatoms, and dinoflagellates) did not exhibit any significant relationship with conductivity (Table S12), so it is unclear which taxa are driving this relationship. More information on the hurricane exclusion analyses can be found in the supplementary information file.
Management implications
Our data indicate that climate change is a critical factor influencing water quality and algal community composition. Local management solutions alone are not enough to mitigate eutrophication and HABs.
The positive relationship between TP and precipitation indicates that erosion reduction could help reduce nutrient loading in the Albemarle Sound. Soil erosion can carry both organic and inorganic P compounds to waterbodies during precipitation events (Howarth et al. 2002; Huttunen et al. 2015). There are multiple methods of reducing erosion that have been employed in coastal ecosystems, like bulkheads, seawalls, and “living shorelines” (Beuzen et al. 2018; Bilkovic et al. 2016; Jackson et al. 2015; Scyphers et al. 2011). Furthermore, previous studies have shown that erosion mitigation measures may be related to water quality improvement (Hunt et al. 2019; Rickson 2014). Our data suggest that the link between erosion reduction and nutrient pollution is worth exploring in the Albemarle Sound.
TP was positively associated with developed and agricultural land use, indicating a potential link between farm and lawn fertilizers, impervious surfaces, and/or yard and pet waste. To reduce phosphorus inputs into the sound, individuals can dispose of their pet and yard waste, rather than leaving it on their lawn or street, where nutrients can leach into the water (Hobbie et al. 2017). Additionally, conserving and expanding greenspace and limiting impervious surface area may help precipitation to percolate into the soil over a greater area, which can reduce runoff (Liu et al. 2014). Increasing green infrastructure, like reservoirs and ponds, has also been shown to effectively limit storm runoff (Liu et al. 2014). Because conventional agricultural methods greatly contribute to nutrient loading (Chakraborty et al. 2017; Glibert et al. 2014), implementing farming methods that use fewer chemical fertilizers and farming practices that reduce nutrient export like cover crops and two-stage ditches, can be beneficial in reducing nutrient inputs and eutrophication (Abdalla et al. 2019; Christopher et al. 2017; Reganold and Wachter 2016).
Protecting SAV can also be a useful method in algal bloom mitigation as macrophytes can serve as nutrient sinks in aquatic environments (Ozimek et al. 1990, Touchette and Burkholder 2000, van Donk et al. 1993). In cases where sources of nutrients on land are unclear, methods that reduce nutrient concentrations from within water bodies may be helpful. For instance, as our data did not indicate a clear source of nitrogen to the sound, protecting SAV could be useful in mitigating cyanobacterial blooms (particularly non-N-fixing genera). Some boat ramps in the study region included informational signs urging visitors to take individual actions to protect aquatic grasses, like avoiding shallow areas in one’s boat (Fig. S9). We suggest implementing more signage like this to educate the public about protecting the waterways they use.
Research limitations and future directions
We faced multiple challenges and limitations in conducting this study. One, sites were selected based on accessibility, which limited the options available. Samples closer to the shore (e.g., sites 1, 2, 9, 20) may be more greatly influenced by waters from upstream rather than land use in their watersheds. Thus, further investigation into how the factors influencing water quality may vary based on a site’s position in the landscape is warranted. Furthermore, the Albemarle region is limited in terms of land use composition. There are not many cities in the study area, and therefore it is difficult to determine the impact of urban development on water quality. The Chesapeake Bay is a comparable estuarine system surrounded by more densely populated cities. There is evidence that the effects of urbanization on water quality in this watershed depend on land use history (e.g., is urban development expanding into forests or agricultural land; Ator et al. 2020). Studying the effects of urban land area in the Chesapeake Bay would improve our understanding of how urbanization affects water quality in brackish water estuaries. We analyzed preserved algal samples rather than fresh samples, which may have impacted the morphology of some algal taxa and complicated taxa identification. Lastly, we were not able to obtain TN data due to COVID-19 restrictions, which limits our knowledge of how N concentration relates to land use and algal community composition.
While understanding the factors that impact the concentrations of various nutrients and algae in the sound provides crucial insight into managing algal blooms, more research should be conducted to better understand the influence of space, time, and land use on algal blooms. Exploring N:P ratios in the sound would be useful in understanding variation in algal composition and growth, especially for cyanobacteria. Sampling runoff directly and sampling at different depths would also provide more information regarding which nutrients and pollutants are entering the sound and their impact on water quality. Furthermore, the variables analyzed in this study did not appear to best explain spatial variation in NO3− concentrations. However, the model of the association between nitrate and proportion agricultural land fits the data just as well as the best model (Table S2; Burnham and Anderson 2002). Future research should examine this relationship further and focus on how other factors, like conductivity, temperature, pH, and even air quality affect nitrate. Lastly, analyzing water quality and algal community composition data in the sound over longer periods of time will help in determining the relationship between climate change, land use, water quality, and algal growth.
Conclusion
Nutrient concentrations in the Albemarle Sound are impacted by varying factors. Nitrate, ammonia, and phosphorus were not significantly associated with the same variables, meaning they may come from different sources. Agricultural fertilizers commonly contain inorganic forms of nitrogen and phosphorus, but nitrate was not significantly associated with proportion of agricultural land use and declined over time in the six sites monitored biweekly even after a large precipitation event, signifying a dilution effect that was not offset from atmospheric N inputs. Total phosphorus showed an opposite relationship with time and increased with proportion farmland. The increase in TP with precipitation may be in part due to erosion. In general, these results illustrate a need for further research into sources of nutrient pollution in the sound.
Algal community composition was influenced by nitrate concentration, water temperature, and precipitation, suggesting that climate change plays an important role in algal growth. Hurricane Isaias demonstrated the impact extreme weather events, leading to a dilution effect with algal richness and potentially influencing the relative biomass of algal taxa. Total biomass increased with water temperature, demonstrating that current warming trends will likely support excessive algal growth at the expense of other aquatic organisms.
Data availability
Data and code will be made available on Mendeley.
References
Abdalla M, Hastings A, Cheng K, Yue Q, Chadwick D, Espenberg M, Truu J, Rees RM, Smith P (2019) A critical review of the impacts of cover crops on nitrogen leaching, net greenhouse gas balance and crop productivity. Glob Change Biol 25(8):2530–2543. https://doi.org/10.1111/gcb.14644
Altman JC, Paerl HW (2012) Composition of inorganic and organic nutrient sources influences phytoplankton community structure in the New River Estuary. North Carolina Aquat Ecol 46(3):269–282. https://doi.org/10.1007/s10452-012-9398-8
ANSP_2001_Diatom Biovolume. (2001). The Academy of Natural Sciences of Drexel University.
APNEP. (n.d.-a). Our Estuary. Albemarle-Pamlico National Estuary Partnership. Retrieved September 25, 2020, from https://apnep.nc.gov/our-estuary
APNEP. (n.d.-b). A to Z in the Albemarle-Pamlico Estuary. Albemarle-Pamlico National Estuary Partnership. Retrieved September 25, 2020, from https://apnep.nc.gov/our-estuary/z-albemarle-pamlico-estuary
APNEP. (n.d.-c). What is the Albemarle-Pamlico Region? Albemarle-Pamlico National Estuary Partnership. Retrieved September 24, 2020, from https://apnep.nc.gov/our-estuary/albemarle-pamlico-region
Arnold TM, Zimmerman RC, Engelhardt KA, Stevenson JC (2017) Twenty-first century climate change and submerged aquatic vegetation in a temperate estuary: the case of Chesapeake Bay. Ecosyst Health Sustain 3(7):1–20. https://doi.org/10.1080/20964129.2017.1353283
Ator SW, Blomquist JD, Webber JS, Chanat JG (2020) Factors driving nutrient trends in streams of the Chesapeake Bay watershed. J Environ Qual 49(4):812–834. https://doi.org/10.1002/jeq2.20101
Bales JD (2003) Effects of Hurricane Floyd Inland Flooding, September–October 1999, on Tributaries to Pamlico Sound North Carolina. Estuaries 26(5):1319–1328. https://doi.org/10.1007/BF02803634
Barko JW, James WF (1998) Effects of submerged aquatic macrophytes on nutrient dynamics, sedimentation, and resuspension. The structuring role of submerged macrophytes in lakes. Springer, pp 197–214
Beer S, Spencer W, Bowes G (1986) Photosynthesis and growth of the filamentous blue-green Alga Lyngbya birgei in relation to its environment. J Aquat Plant Manage 24:61–65
Bennett EM, Carpenter SR, Caraco NF (2001) Human impact on erodable phosphorus and eutrophication: a global perspective: increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. Bioscience 51(3):227–234. https://doi.org/10.1641/0006-3568(2001)051[0227:HIOEPA]2.0.CO;2
Berry MA, Davis TW, Cory RM, Duhaime MB, Johengen TH, Kling GW, Marino JA, Den Uyl PA, Gossiaux D, Dick GJ (2017) Cyanobacterial harmful algal blooms are a biological disturbance to Western Lake Erie bacterial communities. Environ Microbiol 19(3):1149–1162. https://doi.org/10.1111/1462-2920.13640
Beuzen T, Turner IL, Blenkinsopp CE, Atkinson A, Flocard F, Baldock TE (2018) Physical model study of beach profile evolution by sea level rise in the presence of seawalls. Coast Eng 136:172–182. https://doi.org/10.1016/j.coastaleng.2017.12.002
Bhattachan A, Jurjonas MD, Moody AC, Morris PR, Sanchez GM, Smart LS, Taillie PJ, Emanuel RE, Seekamp EL (2018) Sea level rise impacts on rural coastal social-ecological systems and the implications for decision making. Environ Sci Policy 90:122–134. https://doi.org/10.1016/j.envsci.2018.10.006
Bilkovic DM, Mitchell M, Mason P, Duhring K (2016) The role of living shorelines as estuarine habitat conservation strategies. Coast Manag 44(3):161–174. https://doi.org/10.1080/08920753.2016.1160201
Blain CO, Shears NT (2019) Seasonal and spatial variation in photosynthetic response of the kelp Ecklonia radiata across a turbidity gradient. Photosynth Res 140(1):21–38. https://doi.org/10.1007/s11120-019-00636-7
Blomqvist S, Gunnars A, Elmgren R (2004) Why the limiting nutrient differs between temperate coastal seas and freshwater lakes: a matter of salt. Limnol Oceanogr 49(6):2236–2241. https://doi.org/10.4319/lo.2004.49.6.2236
Bratt AR, Finlay JC, Welter JR, Vculek BA, Van Allen RE (2019) Co-limitation by N and P characterizes phytoplankton communities across nutrient availability and land use. Ecosystems. https://doi.org/10.1007/s10021-019-00459-6
Brush GS (2009) Historical land use, nitrogen, and coastal eutrophication: a paleoecological perspective. Estuaries Coasts 32(1):18–28. https://doi.org/10.1007/s12237-008-9106-z
Burgin AJ, Yang WH, Hamilton SK, Silver WL (2011) Beyond carbon and nitrogen: how the microbial energy economy couples elemental cycles in diverse ecosystems. Front Ecol Environ 9(1):44–52. https://doi.org/10.1890/090227
Burnham KP, Anderson DR (2002) AIC Differences, Δi. Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media, Berlin, pp 70–72
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8(3):559–568. https://doi.org/10.1890/1051-0761(1998)008[0559:NPOSWW]2.0.CO;2
Carter, L., A. Terando, K. Dow, K. Hiers, K.E. Kunkel, A. Lascurain, D. Marcy, M. Osland, and P. Schramm (2018). Southeast. In Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II [Reidmiller, D.R., C.W. Avery, D.R. Easterling, K.E. Kunkel, K.L.M. Lewis, T.K. Maycock, and B.C. Stewart (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, pp. 743–808
Census of Agriculture—State and County Profiles—North Carolina. (2017). United States Department of Agriculture National Agricultural Statistics Service. https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/County_Profiles/North_Carolina/index.php
Chakraborty S, Tiwari PK, Sasmal SK, Misra AK, Chattopadhyay J (2017) Effects of fertilizers used in agricultural fields on algal blooms. The Eur Phys J Spec Top 226(9):2119–2133. https://doi.org/10.1140/epjst/e2017-70031-7
Cheung MY, Liang S, Lee J (2013) Toxin-producing cyanobacteria in freshwater: a review of the problems, impact on drinking water safety, and efforts for protecting public health. J Microbiol 51(1):1–10. https://doi.org/10.1007/s12275-013-2549-3
Christian RR, O’Neal JE, Peierls B, Valdes L, Paerl HW (2004) Episodic nutrient loading impacts on eutrophication of the southern Pamlico Sound: The effects of the 1999 hurricanes. Water Resources Research Institute of the University of North Carolina.
Christopher SF, Tank JL, Mahl UH, Yen H, Arnold JG, Trentman MT, Sowa SP, Herbert ME, Ross JA, White MJ, Royer TV (2017) Modeling nutrient removal using watershed-scale implementation of the two-stage ditch. Ecol Eng 108:358–369. https://doi.org/10.1016/j.ecoleng.2017.03.015
Cira EK, Paerl HW, Wetz MS (2016) Effects of nitrogen availability and form on phytoplankton growth in a eutrophied estuary (Neuse River Estuary, NC, USA). PLoS ONE 11(8):1–15. https://doi.org/10.1371/journal.pone.0160663
Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, Lancelot C, Likens GE (2009) Controlling eutrophication: nitrogen and phosphorus. Science 323:1014–1015. https://doi.org/10.1126/science.1167755
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Chang 19(2):292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
de Vernal A, Rochon A, Radi T (2013) Paleoceanography, Biological Proxies|Dinoflagellates. In: Elias SA, Mock CJ (eds) Encyclopedia of quaternary science. Elsevier
Diatom and non-diatom (soft-algae) taxa names. (n.d.). Patrick Center for Environmental Research. Retrieved May 9, 2021, from https://diatom.ansp.org/Taxa.aspx
Elser JJ, Bracken ME, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10(12):1135–1142. https://doi.org/10.1111/j.1461-0248.2007.01113.x
Environmental Defense Fund. (n.d.). Nitrogen management in North Carolina agriculture: Results from five years of on-farm research. https://www.edf.org/sites/default/files/documents/nitrogen-management-north-carolina-results-five-years-on-farm-research.pdf
Environmental systems research institute (ESRI), (2020). ArcGIS Pro 2.5.0.
Fleming LE, Kirkpatrick B, Backer LC, Walsh CJ, Nierenberg K, Clark J, Reich A, Hollenbeck J, Benson J, Cheng YS, Naar J, Pierce R, Bourdelais AJ, Abraham WM, Kirkpatrick G, Zaias J, Wanner A, Mendes E, Shalat S, Baden DG (2011) Review of florida red tide and human health effects. Harmful Algae 10(2):224–233. https://doi.org/10.1016/j.hal.2010.08.006
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53(4):341–356. https://doi.org/10.1641/0006-3568(2003)053[0341:TNC]2.0.CO;2
García F, Freile-Pelegrín Y, Robledo D (2007) Physiological characterization of Dunaliella sp (Chlorophyta, Volvocales) from Yucatan Mexico. Bioresour Technol 98(7):1359–1365. https://doi.org/10.1016/j.biortech.2006.05.051
Glibert PM, Seitzinger S, Heil CA, Burkholder JM, Parrow MW, Codispoti LA, Kelly V (2005) The role of eutrophication in the global proliferation of harmful algal blooms: new perspectives and new approaches. Oceanography 18(2):198–209. https://doi.org/10.5670/oceanog.2005.54
Glibert PM, Maranger R, Sobota DJ, Bouwman L (2014) The Haber Bosch–harmful algal bloom (HB–HAB) link. Environ Res Lett 9(10):1–13. https://doi.org/10.1088/1748-9326/9/10/105001
Harke MJ, Davis TW, Watson SB, Gobler CJ (2016) Nutrient-controlled niche differentiation of western Lake Erie cyanobacterial populations revealed via metatranscriptomic surveys. Environ Sci Technol 50(2):604–615. https://doi.org/10.1021/acs.est.5b03931
Harpole WS, Ngai JT, Cleland EE, Seabloom EW, Borer ET, Bracken ME, Elser JJ, Gruner DS, Hillebrand H, Shurin JB (2011) Nutrient co-limitation of primary producer communities. Ecol Lett 14(9):852–862. https://doi.org/10.1111/j.1461-0248.2011.01651.x
Harrison PJ, Zingone A, Mickelson MJ, Lehtinen S, Ramaiah N, Kraberg AC, Sun J, McQuatters-Gollop A, Jakobsen HH (2015) Cell volumes of marine phytoplankton from globally distributed coastal data sets. Estuar Coast Shelf Sci 162:130–142. https://doi.org/10.1016/j.ecss.2015.05.026
Hawkins PR, Holliday J, Kathuria A, Bowling L (2005) Change in cyanobacterial biovolume due to preservation by Lugol’s Iodine. Harmful Algae 4(6):1033–1043. https://doi.org/10.1016/j.hal.2005.03.001
Hayhoe K, DJ Wuebbles, DR Easterling, DW Fahey, S Doherty, J Kossin, W Sweet, R Vose, M Wehner (2018) Our changing climate. In: Reidmiller DR, CW Avery, DR Easterling, KE Kunkel, KLM Lewis, TK Maycock, and BC Stewart (eds) Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment US, Global Change Research Program, Washington, USA, pp 72–144
Hazen TC, Esch GW (1983) Effect of effluent from a nitrogen fertilizer factory and a pulp mill on the distribution and abundance of Aeromonas hydrophila in Albemarle Sound. North Carolina Appl Environ Microbiol 45(1):31–42. https://doi.org/10.1128/aem.45.1.31-42.1983
Hillebrand H, Dürselen C-D, Kirschtel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic microalgae. J Phycol 35(2):403–424. https://doi.org/10.1046/j.1529-8817.1999.3520403.x
Hobbie SE, Finlay JC, Janke BD, Nidzgorski DA, Millet DB, Baker LA (2017) Contrasting nitrogen and phosphorus budgets in urban watersheds and implications for managing urban water pollution. Proc Natl Acad Sci 114(16):4177–4182. https://doi.org/10.1073/pnas.1618536114
House WA, Jickells TD, Edwards AC, Praska KE, Denison FH (1998) Reactions of phosphorus with sediments in fresh and marine waters. Soil Use Manag 14:139–146. https://doi.org/10.1111/j.1475-2743.1998.tb00632.x
Howarth RW, Sharpley A, Walker D (2002) Sources of nutrient pollution to coastal waters in the United States: implications for achieving coastal water quality goals. Estuaries 25(4):656–676. https://doi.org/10.1007/BF02804898
Huang J, Xu C, Ridoutt BG, Wang X, Ren P (2017) Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J Clean Prod 159:171–179. https://doi.org/10.1016/j.jclepro.2017.05.008
Hunt ND, Hill JD, Liebman M (2019) Cropping system diversity effects on nutrient discharge, soil erosion, and agronomic performance. Environ Sci Technol 53(3):1344–1352. https://doi.org/10.1021/acs.est.8b02193
Huttunen I, Lehtonen H, Huttunen M, Piirainen V, Korppoo M, Veijalainen N, Viitasalo M, Vehviläinen B (2015) Effects of climate change and agricultural adaptation on nutrient loading from Finnish catchments to the Baltic Sea. Sci Total Environ 529:168–181. https://doi.org/10.1016/j.scitotenv.2015.05.055
Jackson NL, Nordstrom KF, Saini S, Smith DR (2015) Influence of configuration of bulkheads on use of estuarine beaches by horseshoe crabs and foraging shorebirds. Environ Earth Sci 74:5749–5758. https://doi.org/10.1007/s12665-015-4592-3
Kemp AC, Horton BP, Culver SJ, Corbett DR, van de Plassche O, Gehrels WR, Douglas BC, Parnell AC (2009) Timing and magnitude of recent accelerated sea-level rise (North Carolina, United States). Geology 37(11):1035–1038. https://doi.org/10.1130/G30352A.1
Le C, Zha Y, Li Y, Sun D, Lu H, Yin B (2010) Eutrophication of lake waters in China: cost, causes, and control. Environ Manage 45(4):662–668. https://doi.org/10.1007/s00267-010-9440-3
Leblanc K, Arístegui J, Armand L, Assmy P, Beker B, Bode A, Breton E, Cornet V, Gibson J, Gosselin M-P (2012) A global diatom database–abundance, biovolume and biomass in the world ocean. Earth Syst Sci Data 4(1):149–165. https://doi.org/10.5194/essd-4-149-2012
Lehman PW (2000) The influence of climate on phytoplankton community biomass in San Francisco Bay Estuary. Limnol Oceanogr 45(3):580–590. https://doi.org/10.4319/lo.2000.45.3.0580
Leytem AB, Dungan RS, Bjorneberg DL, Koehn AC (2011) Emissions of ammonia, methane, carbon dioxide, and nitrous oxide from dairy cattle housing and manure management systems. J Environ Qual 40(5):1383–1394. https://doi.org/10.2134/jeq2009.0515
Lin J, Xie L, Pietrafesa LJ, Ramus JS, Paerl HW (2007) Water quality gradients across Albemarle-Pamlico estuarine system: seasonal variations and model applications. J Coast Res 23(1):213–229. https://doi.org/10.2112/05-0507.1
Liu Z, Wang Y, Li Z, Peng J (2013) Impervious surface impact on water quality in the process of rapid urbanization in Shenzhen. China Environ Earth Sci 68(8):2365–2373. https://doi.org/10.1007/s12665-012-1918-2
Liu W, Chen W, Peng C (2014) Assessing the effectiveness of green infrastructures on urban flooding reduction: a community scale study. Ecol Model 291:6–14. https://doi.org/10.1016/j.ecolmodel.2014.07.012
Lombardo SM (2018) Phenological characterization and effects of environmental attributes on river herring spawning migrations within the Albemarle Sound Watershed. North Carolina State University.
Lopez CB, Jewett EB, Dortch Q, Walton BT, Hudnell HK (2008) Scientific Assessment of Freshwater Harmful Algal Blooms. In: Interagency Working Group on Harmful Algal Blooms, Hypoxia and Human Health of the Joint Subcommittee on Ocean Science and Technology. Washington, DC
Lunt J, Smee DL (2020) Turbidity alters estuarine biodiversity and species composition. ICES J Mar Sci 77(1):379–387. https://doi.org/10.1093/icesjms/fsz214
Mallin MA, Corbett CA (2006) How hurricane attributes determine the extent of environmental effects: multiple hurricanes and different coastal systems. Estuaries Coasts 29(6):1046–1061. https://doi.org/10.1007/BF02798667
Mallin MA, Posey MH, McIver MR, Parsons DC, Ensign SH, Alphin TD (2002) Impacts and recovery from multiple Hurricanes in a Piedmont-coastal plain river system: human development of floodplains greatly compounds the impacts of hurricanes on water quality and aquatic life. Bioscience 52(11):999–1010. https://doi.org/10.1641/0006-3568(2002)052[0999:IARFMH]2.0.CO;2
Moorman, M., Kolb, K. R., & Supak, S. (2014). Estuarine Monitoring Programs in the Albemarle Sound Study Area, North Carolina. US Department of the Interior, US Geological Survey.
National Land Cover Database 2016 (NLCD2016) Legend. (n.d.). Multi-Resolution Land Characteristics (MRLC) Consortium. Retrieved May 9, 2021, from https://www.mrlc.gov/data/legends/national-land-cover-database-2016-nlcd2016-legend
NCDEQ. (2019). NC Counties. NC Dept. of Environmental Quality Online GIS. https://data-ncdenr.opendata.arcgis.com/datasets/nc-counties
Nielson L, Smith CL (2005) Influences on residential yard care and water quality: Tualatin Watershed, Oregon 1. JAWRA J Am Water Resour Assoc 41(1):93–106. https://doi.org/10.1111/j.1752-1688.2005.tb03720.x
North Carolina Climate Office. (2020). NC CRONOS Weather and Climate Database. North Carolina Climate Office. https://climate.ncsu.edu/cronos/?station=312635&temporal=D
Olenina I, Hajdu S, Edler L, Andersson A, Wasmund N, Busch S, Göbel J, Gromisz S, Huseby S, Huttunen M, Jaanus A, Kokkonen, P, Ledaine I, & Niemkiewicz E (2006). Biovolumes and size-classes of phytoplankton in the Baltic Sea. Baltic Sea Environment Proceedings, 1–142
Ozimek T, Gulati RD, van Donk E (1990) Can macrophytes be useful in biomanipulation of lakes? The Lake Zwemlust Ex Hydrobiol 200(1):399–407. https://doi.org/10.1007/BF02530357
Özkan K, Jeppesen E, Søndergaard M, Lauridsen TL, Liboriussen L, Svenning J-C (2013) Contrasting roles of water chemistry, lake morphology, land-use, climate and spatial processes in driving phytoplankton richness in the Danish landscape. Hydrobiologia 710(1):173–187. https://doi.org/10.1007/s10750-011-0996-6
Paerl H (2008) Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater–marine continuum. Cyanobacterial harmful algal blooms: state of the science and research needs. Springer, pp 217–237
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb Ecol 65(4):995–1010. https://doi.org/10.1007/s00248-012-0159-y
Paerl HW, Pinckney JL, Fear JM, Peierls BL (1998) Ecosystem responses to internal and watershed organic matter loading: consequences for hypoxia in the eutrophying Neuse River Estuary, North Carolina, USA. Mar Ecol Prog Ser 166:17–25. https://doi.org/10.3354/meps166017
Paerl HW, Bales JD, Ausley LW, Buzzelli CP, Crowder LB, Eby LA, Fear JM, Go M, Peierls BL, Richardson TL (2001) Ecosystem impacts of three sequential hurricanes (Dennis, Floyd, and Irene) on the United States’ largest lagoonal estuary, Pamlico Sound, NC. Proc Natl Acad Sci 98(10):5655–5660. https://doi.org/10.1073/pnas.101097398
Paerl HW, Valdes LM, Joyner AR, Piehler MF, Lebo ME (2004) Solving problems resulting from solutions: evolution of a dual nutrient management strategy for the eutrophying Neuse River Estuary North Carolina. Environ Sci Technol 38(11):3068–3073. https://doi.org/10.1021/es0352350
Paerl HW, Valdes-Weaver LM, Joyner AR, Winkelmann V (2007) Phytoplankton indicators of ecological change in the eutrophying Pamlico Sound system North Carolina. Ecol Appl 17(sp5):S88–S101. https://doi.org/10.1890/05-0840.1
Paerl HW, Rossignol KL, Hall SN, Peierls BL, Wetz MS (2010) Phytoplankton community indicators of short-and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuaries Coasts 33(2):485–497. https://doi.org/10.1007/s12237-009-9137-0
Passy SI, Legendre P (2006) Are algal communities driven toward maximum biomass? Proc Royal Soc b Biol Sci 273(1601):2667–2674. https://doi.org/10.1098/rspb.2006.3632
Peñuelas J, Poulter B, Sardans J, Ciais P, Van Der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J (2013) Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4(1):1–10. https://doi.org/10.1038/ncomms3934
Phlips EJ, Hendrickson J, Quinlan EL, Cichra M (2007) Meteorological influences on algal bloom potential in a nutrient-rich blackwater river. Freshw Biol 52(11):2141–2155. https://doi.org/10.1111/j.1365-2427.2007.01844.x
Piehler MF, Twomey LJ, Hall NS, Paerl HW (2004) Impacts of inorganic nutrient enrichment on phytoplankton community structure and function in Pamlico Sound, NC, USA. Estuar Coast Shelf Sci 61(2):197–209. https://doi.org/10.1016/j.ecss.2004.05.001
Plaas HE, Paerl RW, Baumann K, Karl C, Popendorf KJ, Barnard MA, Chang NY, Curtis NP, Huang H, Mathieson OL (2022) Harmful cyanobacterial aerosolization dynamics in the airshed of a eutrophic estuary. Sci Total Environ 852:158383. https://doi.org/10.1016/j.scitotenv.2022.158383
Powell N, Shilton A, Pratt S, Chisti Y (2011) Luxury uptake of phosphorus by microalgae in full-scale waste stabilisation ponds. Water Sci Technol 63(4):704–709. https://doi.org/10.2166/wst.2011.116
Reganold JP, Wachter JM (2016) Organic agriculture in the 21st century. Nat Plants 2(2):1–8. https://doi.org/10.1038/nplants.2015.221
Rickson RJ (2014) Can control of soil erosion mitigate water pollution by sediments? Sci Total Environ 468–469:1187–1197. https://doi.org/10.1016/j.scitotenv.2013.05.057
RStudio Team (2019). RStudio: integrated development for R. RStudio, Inc., Boston, MA URL http://www.rstudio.com/.
Rückert GV, Giani A (2004) Effect of nitrate and ammonium on the growth and protein concentration of Microcystis viridis Lemmermann (Cyanobacteria). Braz J Bot 27(2):325–331. https://doi.org/10.1590/S0100-84042004000200011
Savci S (2012) An agricultural pollutant: chemical fertilizer. Int J Environm Sci Dev 3(1):73
Schindler DW (1977) Evolution of phosphorus limitation in lakes. Science 195(4275):260–262. https://doi.org/10.1126/science.195.4275.260
Schmidt S, Diallo II, Derriennic H, Fallou H, Lepage M (2019) Exploring the susceptibility of turbid estuaries to hypoxia as a prerequisite to designing a pertinent monitoring strategy of dissolved oxygen. Front Mar Sci 6:352. https://doi.org/10.3389/fmars.2019.00352
Scyphers SB, Powers SP, Heck KL, Byron D (2011) Oyster reefs as natural breakwaters mitigate shoreline loss and facilitate fisheries. PLoS ONE 6(8):1–12. https://doi.org/10.1371/journal.pone.0022396
Swedish Meteorological and Hydrological Institute (SMHI). (n.d.). Taxonomy. Nordic Microalgae and Aquatic Protozoa. Retrieved May 9, 2021, from http://nordicmicroalgae.org/
Spatharis S, Mouillot D, Danielidis DB, Karydis M, Do Chi T, Tsirtsis G (2008) Influence of terrestrial runoff on phytoplankton species richness-biomass relationships: a double stress hypothesis. J Exp Mar Biol Ecol 362(1):55–62. https://doi.org/10.1016/j.jembe.2008.06.003
Stutter MI, Langan SJ, Cooper RJ (2008) Spatial contributions of diffuse inputs and within-channel processes to the form of stream water phosphorus over storm events. J Hydrol 350(3–4):203–214. https://doi.org/10.1016/j.jhydrol.2007.10.045
Sukenik A, Quesada A, Salmaso N (2015) Global expansion of toxic and non-toxic cyanobacteria: effect on ecosystem functioning. Biodivers Conserv 24(4):889–908. https://doi.org/10.1007/s10531-015-0905-9
Teichberg M, Fox SE, Olsen YS, Valiela I, Martinetto P, Iribarne O, Muto EY, Petti MA, Corbisier TN, Soto-Jiménez M (2010) Eutrophication and macroalgal blooms in temperate and tropical coastal waters: nutrient enrichment experiments with Ulva spp. Glob Change Biol 16(9):2624–2637. https://doi.org/10.1111/j.1365-2486.2009.02108.x
Tester PA, Varnam SM, Culver ME, Eslinger DL, Stumpf RP, Swift RN, Yungel JK, Black MD, Litaker RW (2003) Airborne detection of ecosystem responses to an extreme event: phytoplankton displacement and abundance after hurricane induced flooding in the Pamlico-Albemarle sound system North Carolina. Estuaries 26(5):1353–1364. https://doi.org/10.1007/BF02803637
Touchette BW, Burkholder JM (2000) Review of nitrogen and phosphorus metabolism in seagrasses. J Exp Mar Biol Ecol 250(1–2):133–167. https://doi.org/10.1016/S0022-0981(00)00195-7
Tubiello FN, Salvatore M, Rossi S, Ferrara A, Fitton N, Smith P (2013) The FAOSTAT database of greenhouse gas emissions from agriculture. Environ Res Lett 8(1):1–10. https://doi.org/10.1088/1748-9326/8/1/015009
Ulén BM, Kalisky T (2005) Water erosion and phosphorus problems in an agricultural catchment—need for natural research for implementation of the EU Water framework directive. Environ Sci Policy 8(5):477–484. https://doi.org/10.1016/j.envsci.2005.06.005
Università del Salento. (n.d.). Phytoplankton of Lagoons—Acutodesmus acuminatus. Phytoplankton Bio-Imaging. Retrieved May 9, 2021, from http://phytobioimaging.unisalento.it/Products/AtlasofLagoonPhytoplankton/tabid/326/ctl/ViewAtlante_Dettaglio/mid/847/id_phyto/16070/language/en-US/Default.aspx
U.S. Census Bureau. (2018). Cartographic Boundary Files—Shapefile. The United States Census Bureau. https://www.census.gov/geographies/mapping-files/time-series/geo/carto-boundary-file.html
USGS. (2016). NLCD 2016 Land Cover (CONUS). MRLC. https://www.mrlc.gov/data/nlcd-2016-land-cover-conus
USGS. (n.d.). StreamStats. USGS StreamStats. Retrieved December 11, 2020, from https://streamstats.usgs.gov/ss/
van Donk E, van de Bund WJ (2002) Impact of submerged macrophytes including charophytes on phyto-and zooplankton communities: allelopathy versus other mechanisms. Aquat Bot 72(3–4):261–274. https://doi.org/10.1016/S0304-3770(01)00205-4
van Donk E, Gulati RD, Iedema A, Meulemans JT (1993) Macrophyte-related shifts in the nitrogen and phosphorus contents of the different trophic levels in a biomanipulated shallow lake. In: Hillbricht-Ilkowska A, Pieczyńska E (eds) Nutrient dynamics and retention in land/water ecotones of lowland, temperate lakes and rivers. Springer
Van Houtven G, Van Winkle C, O’Neil M, Matthews K, Sinha P (2016) Economic Valuation of the Albemarle-Pamlico Watershed’s Natural Resources (0214909.000.001). RTI International. https://apnep.nc.gov/media/296/open
Vranješ N, Jovanović M (2011) Cyanotoxins: a dermatological problem. Arch Oncol 19(3–4):64–66. https://doi.org/10.2298/AOO1104064V
Watson SB, Miller C, Arhonditsis G, Boyer GL, Carmichael W, Charlton MN, Confesor R, Depew DC, Höök TO, Ludsin SA (2016) The re-eutrophication of Lake Erie: harmful algal blooms and hypoxia. Harmful Algae 56:44–66. https://doi.org/10.1016/j.hal.2016.04.010
White E, Kaplan D (2017) Restore or retreat? Saltwater intrusion and water management in coastal wetlands. Ecosyst Health Sustain 3(1):1–18. https://doi.org/10.1002/ehs2.1258
Xian G, Crane M, Su J (2007) An analysis of urban development and its environmental impact on the Tampa Bay watershed. J Environ Manage 85(4):965–976. https://doi.org/10.1016/j.jenvman.2006.11.012
Zhang Z, McDonnell KT, Zadok E, Mueller K (2014) Visual correlation analysis of numerical and categorical data on the correlation map. IEEE Trans Visual Comput Graphics 21(2):289–303
Acknowledgements
Thank you to the Davidson Research Initiative for funding this project, Dr. Brad Johnson and Dr. Fuji Lozada for helping advise this project, Dr. Paula Furey for her help with taxon identification and biovolume calculation, Elayna Daniels, Sarah Donovan, Lilly Greene, Ben Heuser, Amanda Lee, and Annika Thesing for their support, Liz, Matt, and Joseph Brentjens for their assistance during fieldwork, Carol and Henry Bean and Ann and David Buchanan for providing a vehicle and kayak, and Jessica Spillman for her assistance in the lab.
Funding
Funding provided by Davidson College Research Initiative and Davidson College Environmental Studies department.
Author information
Authors and Affiliations
Contributions
EB contributed to conceptualization, data collection and analysis, data visualization, writing, and revisions. AB contributed to conceptualization, data analysis, writing, and revisions.
Corresponding author
Additional information
Handling editor: Mao Xiao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brentjens, E.T., Bratt, A.R. Beneath the surface: spatial and temporal trends in water quality and its impacts on algal community composition in the Albemarle Sound, North Carolina. Aquat Ecol 57, 243–262 (2023). https://doi.org/10.1007/s10452-023-10008-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-023-10008-y