Abstract
In this study, an efficient PID control system has been designed for a vacuum pressure swing adsorption (VPSA) process which used silica gel to capture CO2 from dry flue gas. Changes of CO2 composition in feed gas are set as disturbances and vary from 12.0 to 15.0% to make simulation work more closely to reality. Meanwhile, adsorption step time duration was set to change consistently according to the feedback of CO2 purity in product. PID control strategy built in software gPROMS is employed in this paper. The competence of the control system is firstly analyzed by a transient impulse of CO2 content in feed gas (15.0% dropped to 12.0%). Then random pulse is added to test the ability of the control system to reject the unknown disturbances and track the set point. Detailed changes in bed under open and closed-loop are listed and made comparisons. Results demonstrate that the model shows an enhanced performance in the presence of random disturbances under closed-loop control compared with the open-loop operation. And this proves that closed-loop feedback PID control can be used to improve the CO2 capture of VPSA process operations.











Similar content being viewed by others
Abbreviations
- b0 :
-
Adsorption affinity, 1/kPa
- c:
-
Total gas phase concentration, mol/m3
- ci :
-
Gas phase concentration of component i, mol/m3
- Cpg :
-
Constant pressure specific heat of the gas mixture, KJ/(kg K)
- Cps :
-
Specific heat of the adsorbent, KJ/(kg K)
- CV :
-
Valve constant, mol/(KPa s)
- Dax :
-
Axial dispersion coefficient, m2/s
- Dc,i :
-
Effective diffusion coefficient of component i, m2/s
- Dk,i :
-
Knudsen diffusion coefficient of component i, m2/s
- Dm :
-
Molecular diffusion coefficient, m2/s
- dp :
-
Particle pore diameter, m
- DV :
-
Molecular diffusion volume, cm3/mol
- e:
-
Bias of purity
- error:
-
Integral bias of purity
- F:
-
Molar flow of rate, mol/s
- Fin :
-
Molar flow rate into the pump, mol/s
- Fout :
-
Molar flow rate out of the pump, mol/s
- h:
-
Heat transfer coefficient between gas and column wall, W/(m2 K)
- Hb:
-
Bed height, m
- ΔHi :
-
Isosteric heat of adsorption of component i, KJ/mol
- K:
-
Number of cycles
- KC :
-
Proportional gain for PID controller
- Kd :
-
Differential constant for PID controller
- Kg:
-
Effective axial thermal conductivity, W/(m K)
- M:
-
Molecular weight of the gas, kg/mol
- N:
-
Total number of component
- P:
-
Pressure, Pa
- Ps:
-
Set point of purity
- qi:
-
Adsorbed phase concentration of component i, mol/kg
- qm,i :
-
Maximum adsorbed phase concentration in equilibrium with bulk gas of component i, mol/kg
- R:
-
Ideal gas constant, J/(mol K)
- Rb:
-
Bed radius, m
- Rp:
-
Particle radius, m
- t:
-
Time, s
- T:
-
Temperature, K
- Vg:
-
Superficial velocity, m/s
- yi :
-
Molar fraction of component i
- z:
-
Axial direction
- \({\varepsilon _b}\) :
-
Bulk phase porosity
- \({\varepsilon _p}\) :
-
Particle phase porosity
- \({\rho _p}\) :
-
Density of the adsorbent, kg/m3
- \({\rho _g}\) :
-
Density of the gas phase, kg/m3
- \({\eta _p}\) :
-
Work efficiency of compressor and vacuum pump
- \({\tau _i}\) :
-
Proportional gain
- M :
-
Gas viscosity, Pa s
- \({\Gamma}\) :
-
Compressor adiabatic factor
- i:
-
Species
- CCC:
-
CO2 capture and concentration
- CFDM:
-
Central finite difference method
- CO2-EOR:
-
CO2 enhanced oil recovery
- CSS:
-
Cyclic steady state
- DAEs:
-
Differential algebraic equations
- IEA:
-
International Energy Agency
- MOL:
-
The method of lines
- PDEs:
-
The partial differential equations
- r-SQP:
-
Sequential quadratic programming
References
Agarwal, A., Biegler, L.T., Zitney, S.E.: A superstructure-based optimal synthesis of PSA cycles for post-combustion CO2 capture. AiChE J. 56(7), 1813–1828 (2010)
Bitzer, M., Zeite, M.: Design of a nonlinear distributed parameter observer for a pressure swing adsorption plant. J. Process Control 12(4), 533–543 (2002)
Choi, W.K., Kwon, T.I., Yeo, Y.K., Lee, H., Song, H., Na, B.K.: Optimal operation of the pressure swing adsorption process for CO2 recovery. Korean J. Chem. Eng. 20(4), 617–623 (2003)
Delgado, J.A., Uguina, M.A., Sotelo, J.L., Águeda, V.I., Sanz, A., Gómez, P.: Numerical analysis of CO2 concentration and recovery from flue gas by a novel vacuum swing adsorption cycle. Comput. Chem. Eng. 35(6), 1010–1019 (2011)
García, S., Gil, M.V., Pis, J.J., Rubiera, F., Pevida, C.: Cyclic operation of a fixed-bed pressure and temperature swing process for CO2 capture: experimental and statistical analysis. Int. J. Greenh. Gas Control 12(1), 35–43 (2013)
Idem, R., Supap, T., Shi, H., Gelowitz, D., Ball, M., Campbell, C., Tontiwachwuthikul, P.: Practical experience in post-combustion CO2 capture using reactive solvents in large pilot and demonstration plants. Int. J. Greenh. Gas Control 40, 6–25 (2015)
Ishibashi, M., Ota, H., Akutsu, N., Umeda, S., Tajika, M.: Technology for removing carbon dioxide from power plant flue gas by the physical adsorption method. Energy Convers. Manag. 37(6–8), 929–933 (1996)
Jamali, A., Ettehadtavakkol, A.: CO2 storage in residual oil zones: field-scale modeling and assessment. Int. J. Greenh. Gas Control 56, 102–115 (2017)
Khajuria, H., Pistikopoulous, E.N.: Dynamic modeling and explicit/multi-parametric MPC control of pressure swing adsorption systems. J. Process Control 21(1), 151–163 (2011)
Khajuria, H., Pistikopoulous, E.N.: Optimization and control of pressure swing adsorption processes under uncertainty. AIChE J. 59(1), 120–131 (2013)
Ko, D., Siriwardane, R., Biegler, L.T.: Optimization of pressure swing adsorption and fractionated vacuum pressure swing adsorption process for CO2 capture. Ind. Eng. Chem. Res. 44(21), 8084–8094 (2005)
Krishnamurthy, S., Rao, V.R., Guntuka, S., Sharratt, P., Haghpanah, R., Rajendran, A., Amanullah, M., Karimi, I.A., Farooq, S.: CO2 capture from dry flue gas by vacuum swing adsorption: a pilot plant study. AiChE J. 60(5), 1830–1842 (2014)
Li, L., Conway, W., Burns, R., Maeder, M., Puxty, G., Clifford, S., Yu, H.: Investigation of metal ion additives on the suppression of ammonia loss and CO2 absorption kinetics of aqueous ammonia-based CO2 capture. Int. J. Greenh. Gas Control 56, 165–172 (2017)
Liu, Z., Carlos, A., Ping, L., Yu, J., Rodrigues, A.E.: Multi-bed vacuum pressure swing adsorption for carbon dioxide capture from flue gas. Sep. Purif. Technol. 81(3), 307–317 (2011)
Liu, Z., Wang, L., Kong, X., Li, P., Yu, J., Rodrigues, A.E.: Onsite CO2 capture from flue gas by an adsorption process in a coal-fired power plant. Ind. Eng. Chem. Res. 51(21), 7355–7363 (2012)
Na, B.K., Koo, K.K., Eum, H.M., Lee, H., Song, H.: CO2 recovery from flue gas by PSA process using activated carbon. Korean J. Chem. Eng 18(2), 220–227 (2001)
Ren, B., Zhang, L., Huang, H., Ren, S., Chen, G., Zhang, H.: Performance evaluation and mechanisms study of near-miscible CO2 flooding in a tight oil reservoir of Jilin Oilfield China. J. Nat. Gas Sci. Eng. 27, 1796–1805 (2015)
Seggiani, M., Puccini, M., Vitolo, S.: Alkali promoted lithium orthosilicate for CO2 capture at high temperature and low concentration. Int. J. Greenh. Gas Control 17, 25–31 (2013)
Sen, M., Singh, R., Ramachandran, R.: Simulation-based design of an efficient control system for the continuous purification and processing of active pharmaceutical ingredients. J. Pharm. Innov. 9(1), 65–81 (2014)
Shen, C.Z., Liu, Z., Li, P., Yu, J.G.: Two-stage VPSA process for CO2 capture from flue gas using activated carbon beads. Ind. Eng. Chem. Res. 51(13), 5011–5021 (2012)
Shen, S., Bian, Y., Zhao, Y.: Energy-efficient CO2 capture using potassium prolinate/ethanol solution as a phase-changing adsorbent. Int. J. Greenh. Gas Control 56, 1–11 (2017)
Sholl, D.S., Lively, R.P.: Seven chemical separations to change the world. Nature 532(7600), 435–437 (2016)
Shreenath, K., Haghpanah, R., Rajendarn, A., Farroq, S.: Simulation and optimization of a dual-adsorbent, two-bed vacuum swing adsorption process for CO2 capture from wet flue gas. Ind. Eng. Chem. Res. 53(37), 14462–14473 (2014)
Sun, W., Shen, Y., Zhang, D., Yang, H., Ma, H.: A systematic simulation and proposed optimization of the pressure swing adsorption process for N2/CH4 separation under external disturbances. Ind. Eng. Chem. Res. 54(30), 150723150946008 (2015)
Susarla, N., Haghpanah, R., Karimi, A., Farooq, S., Rajendran, A., Tan, L.S.C., Lim, J.S.T.: Energy and cost estimates for capturing CO2 from a dry flue gas using pressure/vacuum swing adsorption. Chem. Eng. Res. Des. 102, 354–367 (2015)
Wang, L., Yang, Y., Shen, W., Kong, X., Li, P., Yu, J., Rodrigues, A.E.: CO2 capture from flue gas in an existing coal-fired power plant by two successive pilot-scale VPSA units. Ind. Eng. Chem. Res. 52(23), 7947–7955 (2013a)
Wang, L., Yang, Y., Shen, W., Kong, X., Li, P., Yu, J., Rodrigues, A.E.: Experimental evaluation of adsorption technology for CO2 capture from flue gas in an existing coal-fired power plant. Chem. Eng. Sci. 101, 615–619 (2013b)
Yan, H., Fu, Q., Zhou, Y., Li, D., Zhang, D.: CO2 capture from dry flue gas by pressure vacuum swing adsorption: a systematic simulation and optimization. Int. J. Greenh. Gas Control 51, 1–10 (2016)
Zhang, P., Tong, J., Jee, Y., Huang, K.: Stabilizing a high-temperature electrochemical silver-carbonate CO2 capture membrane by atomic layer deposition of a ZrO2 overcoat. Chem. Commun. 52(63), 9817–9820 (2016)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Mathematical model
Appendix: Mathematical model
Assumptions for the whole model are listed as follows:
-
1.
Gas phase obey the role of ideal gas.
-
2.
Gas concentration, temperature and pressure only vary in axial direction.
-
3.
Pressure drop along the bed is calculated by Ergun equation.
-
4.
Thermal balance is maintained between gas and solid phase.
-
5.
Kinetics of adsorption abided by linear driving force model.
-
6.
Porosity of both bed and adsorbent particle is uniform along the bed.
The equations used in this paper are listed in follows:
Parameter | Expression |
|---|---|
Component mass balance | \(- \frac{\partial }{{\partial z}}\left( {{\varepsilon _b}{D_{ax}}c\frac{{\partial {y_i}}}{{\partial z}}} \right)+\frac{{\partial \left( {{v_g}{c_i}} \right)}}{{\partial z}}+\left( {{\varepsilon _b}+\left( {1 - {\varepsilon _b}} \right){\varepsilon _p}} \right)\frac{{\partial {c_i}}}{{\partial t}}+{\rho _P}\left( {1 - {\varepsilon _b}} \right)\frac{{\partial {q_i}}}{{\partial t}}=0\) |
Overall mass balance | \(- \frac{\partial }{{\partial z}}\left( {{\varepsilon _b}{D_{ax}}\frac{{\partial c}}{{\partial z}}} \right)+\frac{{\partial \left( {{v_g}c} \right)}}{{\partial z}}+\left( {{\varepsilon _b}+\left( {1 - {\varepsilon _b}} \right){\varepsilon _p}} \right)\frac{{\partial c}}{{\partial t}}+{\rho _P}\left( {1 - {\varepsilon _b}} \right)\frac{{\partial q}}{{\partial t}}=0\) |
Energy balance | \(\begin{gathered} \left\{ {\left[ {{\varepsilon _b}+\left( {1 - {\varepsilon _b}} \right){\varepsilon _p}} \right]\mathop \sum \limits_{{i=1}}^{N} {c_i}\left( {{C_{pg,i}} - R} \right)+\left( {1 - {\varepsilon _b}} \right){\rho _p}\mathop \sum \limits_{{i=1}}^{N} {q_i}\left( {{C_{pg,i}} - R} \right)} \right\}\frac{{\partial T}}{{\partial t}} \hfill \\ +{v_g}{\rho _g}\mathop \sum \limits_{{i=1}}^{N} {C_{pg,i}}\frac{{\partial T}}{{\partial z}}+\left( {1 - {\varepsilon _b}} \right){\varepsilon _p}\mathop \sum \limits_{{i=1}}^{N} {C_{pg,i}}\frac{{\partial {q_i}}}{{\partial t}}\Delta {H_i}+2h\frac{{T - {T_w}}}{{{R_b}}} \hfill \\ - \left( {{\varepsilon _b}+\left( {1 - {\varepsilon _b}} \right){\varepsilon _p}} \right)\frac{{\partial P}}{{\partial t}} - {k_g}\frac{{{\partial ^2}T}}{{\partial {z^2}}}=0 \hfill \\ \end{gathered}\) |
Gas phase momentum balance | \(- \frac{{\partial {\text{P}}}}{{\partial {\text{z}}}}=\frac{{150{{{\upmu}}}{{\left( {1 - {{{{\upvarepsilon}}}_{\text{b}}}} \right)}^2}}}{{{{{\upvarepsilon}}}_{{\text{b}}}^{3}{{\left( {2{{\text{R}}_{\text{p}}}} \right)}^2}}}{{\text{v}}_{\text{g}}}+1.75\frac{{\left( {1 - {{{{\upvarepsilon}}}_{\text{b}}}} \right){{{{\uprho}}}_{\text{g}}}}}{{2{{\text{R}}_{\text{P}}}{{{\upvarepsilon}}}_{{\text{b}}}^{3}}}\left| {{{\text{v}}_{\text{g}}}} \right|{{\text{v}}_{\text{g}}}\) |
Langmuir isotherm | \({q^{*}}=\frac{{{q_{m,1}}{b_i}{P_i}}}{{1+\mathop \sum \nolimits{b_i}{P_i}}}{b_i}=b_{i}^{0}exp\left( { - \frac{{\Delta {H_i}}}{{RT}}} \right)\) |
Linear driving force | \(\frac{{\partial {q_i}}}{{\partial t}}=\frac{{15{D_{C,i}}}}{{R_{p}^{2}}}\left( {q_{i}^{{\text{*}}} - {q_i}} \right)\) |
Diffusion coefficient | \({D_{ax}}=0.73{D_m}+\frac{{{v_g}{R_p}}}{{{\varepsilon _b}\left( {1+9.49\frac{{{\varepsilon _b}{D_m}}}{{2{v_g}{R_p}}}} \right)}}\quad{D_{k,i}}=48.5{D_p}\sqrt {\frac{T}{{{M_i}}}}\) \(D_{m} = \frac{{0.01013T^{{1.75}} \sqrt {\left( {\frac{1}{{M_{A} }} + \frac{1}{{M_{B} }}} \right)} }}{{P\left( {D_{{V,A}}^{{{1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-\nulldelimiterspace} 3}}} + D_{{V,B}}^{{{1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-\nulldelimiterspace} 3}}} } \right)}}\quad D_{{c,i}} = \frac{{\varepsilon _{p} }}{\tau }\frac{{D_{{k,i}} D_{m} }}{{D_{{k,i}} + D_{m} }}\) |
Boundary condition | \({\text{Z}}=0:{v_{g,z}} \geqslant 0,\,{T_z}={T_{in}},\,{y_{i,z}}={y_{in,i}}\,else\frac{{\partial {T_z}}}{{\partial z}}=0,\,\frac{{\partial {y_{i,z}}}}{{\partial z}}=0\) \({\text{Z}}={\text{Hb}}:{v_{g,z}} \leqslant 0,\,{T_z}={T_{out}},\,{y_{i,z}}={y_{out,i}}\,else,\,\frac{{\partial {T_z}}}{{\partial z}}=0,\frac{{\partial {y_{i,z}}}}{{\partial z}}=0\) |
1.1 Sub-models
Sub-models in a VPSA process usually contain buffer tanks, valves and compressors. Dead space in this paper used the same equations as buffer tanks did. Detailed equations are listed as follows:
Buffer tank | Expression |
|---|---|
Energy balance | \(\frac{{\partial T\left( {\mathop \sum \nolimits^{} {n_i}C{p_i}} \right)}}{{\partial t}} - \mathop \sum \limits_{{i=1}}^{N} {F_i}{T_i}\mathop \sum \nolimits^{} {y_k}{C_{pg,i}}+\mathop \sum \limits_{{i=1}}^{N} {F_o}{T_o}\mathop \sum \nolimits^{} {y_k}{C_{pg,i}}=0\) |
Mass balance | \(\frac{{\partial {n_i}}}{{\partial t}} - \mathop \sum \nolimits^{} {F_i}{y_i}+\mathop \sum \nolimits^{} {F_o}{y_o}=0\) |
Valve | \({\text{if}}\,{P_{in}}>{P_{out}}F=\frac{{{C_v}\left( {{P_{in}} - {P_{out}}} \right)}}{{{{10}^6}}}\quad else\,F=0\) |
Compressor and vacuum | \({\text{if}}\,{P_{out}}>{P_{in}}\,{\text{dW}}=\frac{{{P_{out}}{V_{in}}}}{{{\eta _p}}}\frac{\gamma }{{\gamma - 1}}\left[ {{{\left( {\frac{{{P_{out}}}}{{{P_{in}}}}} \right)}^{\frac{{\gamma - 1}}{\gamma }}} - 1} \right]\) \({\text{else}}\,{\text{dW}} =0\quad{W_{cycle}}=\mathop \smallint \limits_{0}^{{{t_{cycle}}}} dW\) |
Performance indicators | \(purit{y_{C{O_2}}}=\frac{{\mathop \smallint \nolimits_{0}^{{{t_{cycle}}}} {F_{out}}{y_{out}}dt}}{{\mathop \smallint \nolimits_{0}^{{{t_{cycle}}}} {F_{out}}dt}}\) \(recover{y_{C{O_2}}}=\frac{{\mathop \smallint \nolimits_{0}^{{{t_{cycle}}}} {F_{out}}{y_{out}}dt}}{{\mathop \smallint \nolimits_{0}^{{{t_{cycle}}}} {F_{in}}{y_{in}}dt}}\) \(productivit{y_{C{O_2}}}=\frac{{3600\mathop \smallint \nolimits_{0}^{{{t_{cycle}}}} {F_{out}}{y_{out}}dt}}{{{t_{cycle}}{W_{ads}}}}\) |
Other data and values for all equations above are listed as follows and remain the same as our previous work. Langmuir non-isothermal model parameters for N2 and CO2 on silica gel were the curving fitting results of pure gas adsorption quantity on silica gel at different temperature and pressure.
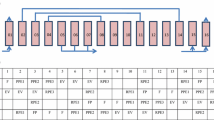
Based on the mathematical models and parameters, the VPSA process is well established under fixed decision variables. In all states analyzed in this paper, all variables in Table 5 remains the same. VAD in table refers to the valve opening (Cv in valve mathematical model) of valve AD (in Fig. 1) and so does the same of other valves. FC1 refers to the flowrate of compressor C1 (in Fig. 1) and so does C2 and vacuum. Valve name that not appeared in the table but used in process means that these valves belongs to the kind of switch valve and do not have the function to adjust the flowrate.
Rights and permissions
About this article
Cite this article
Xing, R., Shi, W., Shen, Y. et al. Vacuum pressure swing adsorption system for N2/CO2 separation in consideration of unstable feed concentration. Adsorption 25, 1147–1158 (2019). https://doi.org/10.1007/s10450-019-00041-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00041-5