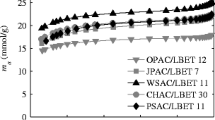
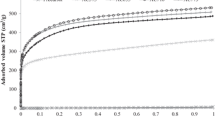
Abstract
The paper presents the results of research into the effects of the additional activation process of the commercial activated carbon WG-12 with KOH, ZnCl2, KOH/ZnCl2 and K2CO3 as activating agents on the formation of the porous structure and the adsorptive properties of that material. The numerical analyses were carried out on the basis of the isotherms of nitrogen adsorption with the use of the method based on the Brunauer-Emmett-Teller, the Dubinin-Radushkevich equations, the non-local and the quenched solid density functional theories as well as the LBET method with the unique fast multivariate procedure of porous structure identification and the new LBET class adsorption models. Also, the research in question yielded information regarding the usefulness of the said methods of carbonaceous adsorbent porous structure description for practical technological applications and scientific research, as well as the possibilities to make practical use of the research results.

Similar content being viewed by others
Abbreviations
- mA :
-
The total adsorption (mmol/g), m hA - the number of primary sites at the adsorbate pores
- VhA :
-
The volume of a space accessible for the first layer adsorption (cm3/g)
- θ kj :
-
The coverage ratio of j th layer at k th type clusters
- θ :
-
The coverage ratio of layers n >1
- π :
-
The relative pressure
- α :
-
The geometrical parameter of the microporous structure
- β :
-
The average number of sites provided by (n-1)-th layer for the n-th layer, n 2,k, averaged over all clusters
- Q A and Q C :
-
The adsorption energies at the first (Q A ) and higher layers (Q C ) (J/mol)
- R :
-
The gas constant
- T :
-
Temperature
- B Ak , B fk :
-
The energy parameters
References
Bajaj, P., Dhawan, A.: PAN-based activated carbon fibres: production, characterization and applications. Indian J. Fibre Text. Res. 22, 222–235 (1997)
Bansal, R.C., Donnet, J.B., Stoeckli, R.: Active Carbon. Marcel Dekker, New York (1988)
Benaddi, H., Bandosz, J., Jagiello, J., Schwarz, J.A., Rouzaud, J.N., Legras, D., Béguin, F.: Surface functionality and porosity of activated carbons obtained from chemical activation of wood. Carbon. 38(5), 669–674 (2000)
Bhatia, S.K.: Density functional theory analysis of the influence of pore wall heterogeneity on adsorption in carbons. Langmuir. 18(18), 6845–6856 (2002)
Brunauer, S., Emmett, P.H., Teller, E.: Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938)
Chmiel, G., Lajtar, L., Sokołowski, S., Patrykiejew, A.: Adsorption in energetically heterogeneous slit-like pores: comparison of density functional theory and computer simulations. J. Chem. Soc. Faraday Trans. 90, 1153–1156 (1994)
Cuerda-Correa, E.M., Díaz-Díez, M.A., Macías-García, A., Gañán-Gómez, J.: Preparation of activated carbons previously treated with sulfuric acid: a study of their adsorption capacity in solution. Appl. Surf. Sci. 252(17), 6042–6045 (2006)
Do, D.D., Do, H.D.: Modeling of adsorption on nongraphitized carbon surface: GCMC simulation studies and comparison with experimental data. J. Phys. Chem. B. 110(35), 17531–17538 (2006)
Dubinin, M.M.: The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 60, 235–241 (1960)
Dubinin, M.M., Polyakov, N.S., Kataeva, L.I.: Basic properties of equations for physical vapor adsorption in micropores of carbon adsorbents assuming a normal micropore distribution. Carbon. 29(4–5), 481–488 (1991)
Duda, J.T., Kwiatkowski, M., Milewska-Duda, J.: Computer modeling and analysis of heterogeneous structures of microporous carbonaceous materials. J. Mol. Model. 11(4–5), 416–430 (2005)
Foo, K.Y., Hameed, B.H.: Microwave assisted preparation of activated carbon from pomelo skin for the removal of anionic and cationic dyes. Chem. Eng. J. 173(20), 385–390 (2011)
Foo, K.Y., Hameed, B.H.: Mesoporous activated carbon from wood sawdust by K2CO3 activation using microwave heating. Bioresour. Technol. 111, 425–432 (2012)
Franklin, R.E.: Crystallite growth in graphitizing and nongraphitizing carbons. Proc. R Soc. A 209 196–218 (1951)
Fripiat, J.J., Gatineau, L., van Damme, H.: Multilayer physical adsorption on fractal surfaces. Langmuir. 2(5), 562–567 (1986)
Glonek, K., Sreńscek-Nazzal, J., Narkiewicz, U., Morawski, A.W., Wróbel, R.J., Michalkiewicz, B.: Preparation of activated carbon from beet molasses and TiO2 as the adsorption of CO2. Acta Phys. Polonica A. 129(3), 158–161 (2016)
Grześkowiak, M., Wróbel, R.J., Moszyński, D., Mozia, S., Grzechulska-Damszel, J., Morawski, A. W., Przepiórski, J.: TiO2 supported on quartz wool for photocatalytic oxidation of hydrogen sulphide. Adsorpt. Sci. Technol. 32(10), 765–772 (2014).
ISO 15901-3.: Pore size distribution and porosity of solid materials by mercury porosimetry and gas adsorption, Part 3: Analysis of Micropores by Gas Adsorption, International Organization for Standardization (2007)
Jagiello, J., Ansón, A., Martínez, M.T.: DFT-based prediction of high-pressure H2 adsorption on porous carbons at ambient temperatures from low-pressure adsorption at 77 K. J. Phys. Chem. B. 110, 4531–4534 (2006)
Jagiełło, J., Thommes, M.M.: Comparison of DFT characterization methods based on N2, Ar, CO2, and H2 adsorption applied to carbons with various pore size distributions. Carbon. 42, 1227–1232 (2004)
Kante, K., Nieto-Delgado, C., Rangel-Mendez, J.R., Bandosz, T.J.: Spent coffee-based activated carbon: specific surface features and their importance for H2S separation process. J. Hazard. Mater. 201–202, 141–147 (2011)
Kumar, A., Jena, H.M.: Preparation and characterization of high surface area activated carbon from Fox nut (Euryale ferox) shell by chemical activation with H3PO4. Results Phys. 6, 651–658 (2016)
Kwiatkowski, M.: Computer analysis of microporous structure by employing the LBET class models with various variants of the adsorption energy distribution in comparison to the classical equations. Langmuir. 23, 2569–2581 (2007)
Kwiatkowski, M.: Computer analyses of new numerical methods for the description of adsorption process and the reliability of identification of microporous structure parameters. J. Mol. Model. 14, 183–200 (2008)
Kwiatkowski, M.: Use of fast multivariant identification of the parameters of adsorption systems to study the impact of activating agent on microporous structure formation during activation. J. Colloid Interface Sci. 340(1), 1–7 (2009)
Kwiatkowski, M., Broniek, E.: Application of the LBET class adsorption models to the analysis of microporous structure of the active carbons produced from biomass by chemical activation with the use of potassium carbonate. Colloid Surf. A. 427, 47–52 (2013)
Kwiatkowski, M., Wiśniewski, M., Rychlicki, G.: The numerical analysis of the spherical carbon adsorbents obtained from ion-exchange resins in one-step steam pyrolysis. Appl. Surf. Sci. 259, 13–20 (2012)
Kwiatkowski, M., Policicchio, A., Seredych, M., Bandosz, T.J.: Evaluation of CO2 interactions with S-doped nanoporous carbon and its composites with a reduced GO: Effect of surface features on an apparent physical adsorption mechanism. Carbon. 98, 250–258 (2016)
Kwiatkowski, M., Fierro, V., Celzard, A.: Numerical studies of the effects of process conditions on the development of the porous structure of adsorbents prepared by chemical activation of lignin with alkali hydroxides. J. Colloid Interface Sci. 486, 277–286 (2017)
Landers, J., Gor, G.Y., Neimark, A.V.: Density functional theory methods for characterization of porous materials. Colloids Surf. A. 437, 3–32 (2013)
Lastoskie, C.M., Gubbins, K.E.: Characterization of porous materials using molecular theory and simulation. Adv. Chem. Eng. 28, 203–250 (2001)
Lastoskie, C., Gubbins, K.E., Quirke, N.: Pore size distribution analysis of microporous carbons: a density functional theory approach. J. Phys. Chem. 97, 4786–4796 (1993)
Marsh, H., Rodríguez-Reinoso, F.: Activated Carbon. Elsevier, Oxford (2006)
Młodzik, J., Sreńscek-Nazzal, J., Narkiewicz, U., Morawski, A.W., Wróbel, R.J., Michalkiewicz, B.: Activated carbons from molasses as CO2 sorbents. Acta Phys. Polonica A. 129(3), 402–404 (2016)
Neimark, A.V., Ravikovitch, P.I., Vishnyakov, A.: Bridging scales from molecular simulations to classical thermodynamics: density functional theory of capillary condensation in nanopores. J. Phys. 15, 347–365 (2003)
Neimark, A.V., Lin, Y., Ravikovitch, P.I., Thommes, M.: Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon. 47(7), 1617–1628 (2009)
Olivier, J.P.: Improving the models used for calculating the size distribution of micropore volume of activated carbons from adsorption data. Carbon. 36(10), 1469–1472 (1998)
Olivier, J.P., Conklin, W.B., Szombathely, M.V.: Determination of pore size distribution from density functional theory: a comparison of nitrogen and argon results. Stud. Surf. Sci. Catal. 87, 81–89 (1994)
Ravikovitch, P.I., Neimark, A.V.: Unified approach to pore size characterization of microporous carbonaceous materials from N2, Ar, and CO2 adsorption isotherms. Langmuir. 16, 2311–2320 (2000)
Ravikovitch, P.I., Neimark, A.V.: Density functional theory model of adsorption on amorphous and microporous silica materials. Langmuir. 22(26), 11171–11179 (2006)
Salame, I.I., Bandosz, T.J.: Comparison of the surface features of two wood-based activated carbons. Ind. Eng. Chem. Res. 39(2), 301–306 (2000)
Seaton, N., Walton, J., Quirke, N.: A new analysis method for the determination of the pore size distribution of porous carbons from nitrogen adsorption measurements. Carbon. 27(6), 853–861 (1989)
Sentorun-Shalaby, C., Ucak-Astarlioglu, M.G., Artok, L., Sarici, C.: Preparation and characterization of activated carbons by onestep steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater. 88(1–3), 126–134 (2006)
Shahsavand, A., Ahmadpour, A.: Application of optimal RBF neural networks for optimization and characterization of porous materials. Comput. Chem. Eng. 29(10), 2134–2143 (2005)
Sreńscek-Nazzal, J., Kamińska, W., Michalkiewicz, B., Koren, Z.C.: Production, characterization and methane storage potential of KOH activated carbon from sugarcane molasses. Ind. Crops. Prod. 47, 153–159 (2013)
Sreńscek-Nazzal, J., Narkiewicz, U., Morawski, A.W., Wróbel, R.J., Michalkiewicz, B.: Comparison of optimized isotherm models and error functions for carbon dioxide adsorption on activated carbon. J. Chem. Eng. Data. 60, 3148–3158 (2015)
Sreńscek-Nazzal, J., Narkiewicz, U., Morawski, A.W., Wróbel, R.J., Gesikiewicz-Puchalska, A., Michalkiewicz, B.: Modification of commercial activated carbons for CO2 adsorption. Acta Phy. Polonica A. 129(3), 394–401 (2016)
Sun, J.: Pore size distribution model derived from a modified DR equation and simulated pore filling for nitrogen adsorption at 77 K. Carbon. 40, 1051–1062 (2002)
Thommes, M., Smarsly, B., Groenewolt, M., Ravikovitch, P.I., Neimark, A.V.: Adsorption hysteresis of nitrogen and argon in pore networks and characterization of novel micro- and mesoporous silicas. Langmuir. 22(2), 756–764 (2006)
Thomson, K.T., Gubbins, K.E.: Modeling structural morphology of microporous carbons by Reverse Monte Carlo. Langmuir. 16(13), 5761–5773 (2000)
Ustinov, E.A., Do, D.D., Fenelonov, V.B.: Pore size distribution analysis of activated carbons: Application of density functional theory using nongraphitized carbon black as a reference system. Carbon. 44(4), 653–663 (2006)
Vargas, D.P., Giraldo, L., Moreno-Piraján, J.C.: Carbon dioxide and methane adsorption at high pressure on activated carbon materials. Adsorption. 19(6), 1075–1082 (2013)
Vargas, D.P., Giraldo, L., Moreno-Piraján, J.C.: Calorimetric study of the CO2 adsorption on carbon materials. J. Therm. Anal. Calorim. 117, 1299–1309 (2014)
Acknowledgements
The research is led within the AGH University of Science and Technology Grant No. 11.11.210.217.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kwiatkowski, M., Sreńscek-Nazzal, J. & Michalkiewicz, B. An analysis of the effect of the additional activation process on the formation of the porous structure and pore size distribution of the commercial activated carbon WG-12. Adsorption 23, 551–561 (2017). https://doi.org/10.1007/s10450-017-9867-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-017-9867-4