Abstract
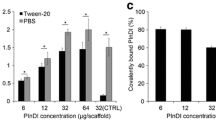
Polycaprolactone (PCL) fiber scaffolds are attractive, albeit inert, substrates for ligament regeneration, that may be improved by incorporating trophic factors to guide tissue remodeling in vivo. In particular, immobilization of bone morphogenic protein-2 (BMP-2) to the scaffold surface may facilitate rapid and robust integration of the scaffold with adjacent bone tissues. As a first step toward testing this, model PCL surfaces were modified by the addition of heparin (Hep) and BMP-2 to facilitate osteoblastic differentiation. Specifically, Hep was combined with PCL at 0, 0.5, and 1 wt% (denoted as PCL, PCL–0.5Hep, and PCL–1Hep), cast into films, and then BMP-2 was immobilized to surfaces by either adsorption and covalent conjugation. Here, BMP-2 concentration increased systematically with incorporation of Hep, and higher concentrations were achieved by covalent conjugation. Next, blends were electrospun to form thin meshes with fiber diameters of 0.92, 0.62, and 0.54 μm for PCL, PCL–0.5Hep, and PCL–1Hep, respectively. Mesenchymal stem cells (MSCs) had no difficulty attaching to and proliferating on all meshes. Lastly, PCL–1Hep meshes were prepared with adsorbed or covalently conjugated BMP-2 and cultured with MSCs in the absence of osteogenic factors. Under these conditions, alkaline phosphatase activity and deposition of bone sialoprotein, osteopontin, and calcium minerals—markers of osteoblastic differentiation—were significantly higher on surfaces with immobilized BMP-2. Together, these data indicate that covalent immobilization of trophic factors confers bioactivity to scaffolds, which may be applied in a spatially controlled manner for ligament regeneration and bone integration.








Similar content being viewed by others
References
Beederman, M., J. D. Lamplot, G. Nan, J. Wang, X. Liu, L. Yin, R. Li, W. Shui, H. Zhang, S. H. Kim, W. Zhang, J. Zhang, Y. Kong, S. Denduluri, M. R. Rogers, A. Pratt, R. C. Haydon, H. H. Luu, J. Angeles, L. L. Shi, and T. C. He. BMP signaling in mesenchymal stem cell differentiation and bone formation. J. Biomed. Sci. Eng. 6:32–52, 2013.
Bhattacharjee, P., D. Naskar, T. K. Maiti, D. Bhattacharya, and S. C. Kundu. Investigating the potential of combined growth factors delivery, from non-mulberry silk fibroin grafted poly(ɛ-caprolactone)/hydroxyapatite nanofibrous scaffold, in bone tissue engineering. Appl. Mater. Today 5:52–67, 2016.
Budiraharjo, R., K. G. Neoh, and E. T. Kang. Enhancing bioactivity of chitosan film for osteogenesis and wound healing by covalent immobilization of BMP-2 or FGF-2. J. Biomater. Sci. Polym. Ed. 24:645–662, 2013.
Cao, L., Y. Yu, J. Wang, J. A. Werkmeister, K. M. McLean, and C. Liu. 2-N, 6-O-sulfated chitosan-assisted BMP-2 immobilization of PCL scaffolds for enhanced osteoinduction. Mater. Sci. Eng. C 74:298–306, 2017.
de Mille, P., and J. Osmak. Performance: bridging the gap after ACL surgery. Curr. Rev. Musculoskelet. Med. 10:297–306, 2017.
De Witte, T. M., L. E. Fratila-Apachitei, A. A. Zadpoor, and N. A. Peppas. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen. Biomater. 5:197–211, 2018.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126:677–689, 2006.
Gandhi, N. S., and R. L. Mancera. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim. Biophys. Acta 1374–1381:2012, 1824.
Gandhi, N. S., and R. L. Mancera. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 72:455–482, 2008.
Gentile, P., K. McColgan-Bannon, N. C. Gianone, F. Sefat, K. Dalgarno, and A. M. Ferreira. Biosynthetic PCL-graft-collagen bulk material for tissue engineering applications. Materials (Basel) 10:693, 2017.
Jia, L., M. P. Prabhakaran, X. Qin, D. Kai, and S. Ramakrishna. Biocompatibility evaluation of protein-incorporated electrospun polyurethane-based scaffolds with smooth muscle cells for vascular tissue engineering. J. Mater. Sci. 48:5113–5124, 2013.
Kang, S., J. S. Yoon, J. Y. Lee, H.-J. Kim, K. Park, and S. E. Kim. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohydr. Polym. 209:372–381, 2019.
Karageorgiou, V., L. Meinel, S. Hofmann, A. Malhotra, V. Volloch, and D. Kaplan. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. A 71:528–537, 2004.
Kawakami, Y., K. Takayama, T. Matsumoto, Y. Tang, B. Wang, Y. Mifune, J. H. Cummins, R. J. Warth, R. Kuroda, M. Kurosaka, F. H. Fu, and J. Huard. Anterior cruciate ligament-derived stem cells transduced with BMP2 accelerate graft–bone integration after ACL reconstruction. Am. J. Sports Med. 45:584–597, 2017.
Keselowsky, B. G., D. M. Collard, and A. J. Garcia. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials 25:5947–5954, 2004.
Keselowsky, B. G., D. M. Collard, and A. J. García. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J. Biomed. Mater. Res. A 66:247–259, 2003.
Kim, K., Y. K. Luu, C. Chang, D. Fang, B. S. Hsiao, B. Chu, and M. Hadjiargyrou. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 98:47–56, 2004.
Li, C., C. Vepari, H. J. Jin, H. J. Kim, and D. L. Kaplan. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 27:3115–3124, 2006.
Liu, S., Y. Liu, L. Jiang, Z. Li, S. Lee, C. Liu, J. Wang, and J. Zhang. Recombinant human BMP-2 accelerates the migration of bone marrow mesenchymal stem cells via the CDC42/PAK1/LIMK1 pathway in vitro and in vivo. Biomater. Sci. 7:362–372, 2018.
Mall, N. A., P. N. Chalmers, M. Moric, M. J. Tanaka, B. J. Cole, B. R. Bach, Jr., and G. A. Paletta, Jr. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am. J. Sports Med. 42:2363–2370, 2014.
Nam, J., J. Johnson, J. J. Lannutti, and S. Agarwal. Modulation of embryonic mesenchymal progenitor cell differentiation via control over pure mechanical modulus in electrospun nanofibers. Acta Biomater. 7:1516–1524, 2011.
Rodeo, S. A., K. Suzuki, X.-H. Deng, J. Wozney, and R. F. Warren. Use of recombinant human bone morphogenetic protein-2 to enhance tendon healing in a bone tunnel. Am. J. Sports Med. 27:476–488, 1999.
Samavedi, S., S. A. Guelcher, A. S. Goldstein, and A. R. Whittington. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament–bone interface. Biomaterials 33:7727–7735, 2012.
Song, F., D. Jiang, T. Wang, Y. Wang, F. Chen, G. Xu, Y. Kang, and Y. Zhang. Mechanical loading improves tendon–bone healing in a rabbit anterior cruciate ligament reconstruction model by promoting proliferation and matrix formation of mesenchymal stem cells and tendon cells. Cell. Physiol. Biochem. 41:875–889, 2017.
Spalazzi, J. P., S. B. Doty, K. L. Moffat, W. N. Levine, and H. H. Lu. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng. 12:3497–3508, 2006.
Uciechowska-Kaczmarzyk, U., S. Babik, F. Zsila, K. K. Bojarski, T. Beke-Somfai, and S. A. Samsonov. Molecular dynamics-based model of VEGF-A and its heparin interactions. J. Mol. Graph. Model. 82:157–166, 2018.
Zhang, J., J. Wang, Y. Wei, C. Gao, X. Chen, W. Kong, D. Kong, and Q. Zhao. ECM-mimetic heparin glycosaminoglycan-functionalized surface favors constructing functional vascular smooth muscle tissue in vitro. Colloids Surf. B 146:280–288, 2016.
Zong, X., K. Kim, D. Fang, S. Ran, B. S. Hsiao, and B. Chu. Structure and process relationship of electrospun bioabsorbable nanofiber membranes. Polymer 43:4403–4412, 2002.
Acknowledgments
The authors would like to thank Dr. William Ducker for the use of his contact angle goniometer, Dr. Rafael Davalos for the use of his spin coater, and Dr. Vincent Wang for the use of his mechanic testing frame. This work was supported by the Interdisciplinary Graduate Educational Program in Regenerative Medicine and the Department of Chemical Engineering at Virginia Tech.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gadalla, D., Goldstein, A.S. Improving the Osteogenicity of PCL Fiber Substrates by Surface-Immobilization of Bone Morphogenic Protein-2. Ann Biomed Eng 48, 1006–1015 (2020). https://doi.org/10.1007/s10439-019-02286-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-019-02286-1