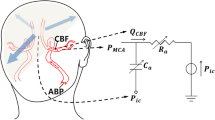
Abstract
Precise management of patients with cerebral diseases often requires intracranial pressure (ICP) monitoring, which is highly invasive and requires a specialized ICU setting. The ability to noninvasively estimate ICP is highly compelling as an alternative to, or screening for, invasive ICP measurement. Most existing approaches for noninvasive ICP estimation aim to build a regression function that maps noninvasive measurements to an ICP estimate using statistical learning techniques. These data-based approaches have met limited success, likely because the amount of training data needed is onerous for this complex applications. In this work, we discuss an alternative strategy that aims to better utilize noninvasive measurement data by leveraging mechanistic understanding of physiology. Specifically, we developed a Bayesian framework that combines a multiscale model of intracranial physiology with noninvasive measurements of cerebral blood flow using transcranial Doppler. Virtual experiments with synthetic data are conducted to verify and analyze the proposed framework. A preliminary clinical application study on two patients is also performed in which we demonstrate the ability of this method to improve ICP prediction.
Similar content being viewed by others
References
Andrews, P. J., G. Citerio, L. Longhi, K. Polderman, J. Sahuquillo, P. Vajkoczy, N.-I. Care, E. M. N. S. of the European Society of Intensive Care Medicine et al. NICEM consensus on neurological monitoring in acute neurological disease. Intensive Care Med. 34:1362–1370, 2008.
Arnold, A., C. Battista, D. Bia, Y. Z. German, R. L. Armentano, H. Tran, and M. S. Olufsen. Uncertainty quantification in a patient-specific one-dimensional arterial network model: EnKF-based inflow estimator. J. Verif. Valid. Uncertain. Quantif. 2:011002, 2017.
Arroyo-Palacios, J., M. Rudz, R. Fidler, W. Smith, N. Ko, S. Park, Y. Bai, and X. Hu. Characterization of shape differences among ICP pulses predicts outcome of external ventricular drainage weaning trial. Neurocritical Care 25:424–433, 2016.
Asiedu, D. P., K.-J. Lee, G. Mills, and E. E. Kaufmann. A review of non-invasive methods of monitoring intracranial pressure. J. Neurol. Res. 4:1–6, 2014.
Bertoglio, C., P. Moireau, and J.-F. Gerbeau. Sequential parameter estimation for fluid–structure problems: Application to hemodynamics. Int. J. Numer. Methods Biomed. Eng. 28:434–455, 2012.
Brandi, G., M. Béchir, S. Sailer, C. Haberthür, R. Stocker, and J. F. Stover. Transcranial color-coded duplex sonography allows to assess cerebral perfusion pressure noninvasively following severe traumatic brain injury. Acta Neurochir. 152:965–972, 2010.
Cardim, D., C. Robba, M. Bohdanowicz, J. Donnelly, B. Cabella, X. Liu, M. Cabeleira, P. Smielewski, B. Schmidt, and M. Czosnyka. Non-invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: is it possible? Neurocritical Care. 25:473–491, 2016.
Connolly, M., P. Vespa, N. Pouratian, N. R. Gonzalez, and X. Hu. Characterization of the relationship between intracranial pressure and electroencephalographic monitoring in burst-suppressed patients. Neurocritical Care 22:212–220, 2015.
Dennis, B., J. M. Ponciano, S. R. Lele, M. L. Taper, and D. F. Staples. Estimating density dependence, process noise, and observation error. Ecol. Monogr. 76:323–341, 2006.
Ghajar, J. Traumatic brain injury. The Lancet 356:923–929, 2000.
Giller, C. A. A bedside test for cerebral autoregulation using transcranial doppler ultrasound. Acta Neurochir. 108:7–14, 1991.
Hickman, K., B. Mayer, and M. Muwaswes. Intracranial pressure monitoring: review of risk factors associated with infection. Heart & Lung: The J. Criti. Care 19:84–90, 1990.
Hu, X., N. Gonzalez, and M. Bergsneider. Steady-state indicators of the intracranial pressure dynamic system using geodesic distance of the icp pulse waveform. Physiol. Meas. 33:2017, 2012.
Hu, X., V. Nenov, M. Bergsneider, T. C. Glenn, P. Vespa, and N. Martin. Estimation of hidden state variables of the intracranial system using constrained nonlinear Kalman filters. IEEE Trans. Biomed. Eng. 54:597–610, 2007.
Hu, X., V. Nenov, M. Bergsneider, and N. Martin. A data mining framework of noninvasive intracranial pressure assessment. Biomed. Signal Process. Control 1:64–77, 2006.
Hu, X., P. Xu, F. Scalzo, P. Vespa, and M. Bergsneider. Morphological clustering and analysis of continuous intracranial pressure. IEEE Trans. Biomed. Eng. 56:696–705, 2009.
Hughes, T. J. and J. Lubliner. On the one-dimensional theory of blood flow in the larger vessels. Math. Biosci. 18:161–170, 1973.
Iglesias, M. A. A regularizing iterative ensemble Kalman method for PDE-constrained inverse problems. Inverse Probl. 32:025002, 2016.
Iglesias, M. A., K. J. Law, and A. M. Stuart. Ensemble Kalman methods for inverse problems. Inverse Probl. 29:045001, 2013.
Iman, R. L. Latin hypercube sampling. Encyclopedia of quantitative risk analysis and assessment , 2008.
Itu, L., P. Sharma, C. Suciu, F. Moldoveanu, and D. Comaniciu. Personalized blood flow computations: A hierarchical parameter estimation framework for tuning boundary conditions. Int. J. Numer Methods Biomed. Eng. 33:e02803, 2017.
Kashif, F. M., G. C. Verghese, V. Novak, M. Czosnyka, and T. Heldt. Model-based noninvasive estimation of intracranial pressure from cerebral blood flow velocity and arterial pressure. Sci. Transl. Med. 4:129ra44–129ra44, 2012.
Kim, S., R. Hamilton, S. Pineles, M. Bergsneider, and X. Hu. Noninvasive intracranial hypertension detection utilizing semisupervised learning. IEEE Trans. Biomed. Eng. 60:1126–1133, 2013.
Kim, S., F. Scalzo, M. Bergsneider, P. Vespa, N. Martin, and X. Hu. Noninvasive intracranial pressure assessment based on a data-mining approach using a nonlinear mapping function. IEEE Trans. Biomed. Eng. 59:619–626, 2012.
Lal, R., F. Nicoud, E. Le Bars, J. Deverdun, F. Molino, V. Costalat, and B. Mohammadi. Non invasive blood flow features estimation in cerebral arteries from uncertain medical data. Ann. Biomed. Eng. 45:2574–2591, 2017.
Linninger, A. A., K. Tangen, C.-Y. Hsu, and D. Frim. Cerebrospinal fluid mechanics and its coupling to cerebrovascular dynamics. Annu. Rev. Fluid Mech. 48:219–257, 2016.
Linninger, A. A., M. Xenos, B. Sweetman, S. Ponkshe, X. Guo, and R. Penn. A mathematical model of blood, cerebrospinal fluid and brain dynamics. J. Math. Biol. 59:729–759, 2009.
Marsden, A. L. Optimization in cardiovascular modeling. Annu. Rev. Fluid Mech. 46:519–546, 2014.
Moireau, P., C. Bertoglio, N. Xiao, C. A. Figueroa, C. Taylor, D. Chapelle, and J.-F. Gerbeau. Sequential identification of boundary support parameters in a fluid-structure vascular model using patient image data. Biomech. Model. Mechanobiol. 12:475–496, 2013.
Moré, J. J. The Levenberg-Marquardt algorithm: implementation and theory. In: Numerical Analysis, edited by G. A. Watson, Berlin: Springer, pp. 105–116, 1978.
Pant, S., C. Corsini, C. Baker, T.-Y. Hsia, G. Pennati, and I. E. Vignon-Clementel. Data assimilation and modelling of patient-specific single-ventricle physiology with and without valve regurgitation. J. Biomech. 49:2162–2173, 2016.
Piper, I. R., K. Chan, I. R. Whittle, and J. D. Miller. An experimental study of cerebrovascular resistance, pressure transmission, and craniospinal compliance. Neurosurgery 32:805–816, 1993.
Piper, I. R., J. D. Miller, N. M. Dearden, J. R. Leggate, and I. Robertson. Systems analysis of cerebrovascular pressure transmission: an observational study in head-injured patients. J. Neurosurg. 73:871–880, 1990.
Ryu, J., X. Hu, and S. C. Shadden. A coupled lumped-parameter and distributed network model for cerebral pulse-wave hemodynamics. J. Biomech. Eng. 137:101009, 2015.
Ryu, J., N. Ko, X. Hu, and S. C. Shadden. Numerical investigation of vasospasm detection by extracranial blood velocity ratios. Cerebrovasc. Dis. 43:214–222, 2017.
Serban, R. and A. C. Hindmarsh. CVODES: the sensitivity-enabled ODE solver in SUNDIALS. In: ASME 2005 international design engineering technical conferences and computers and information in engineering conference. American Society of Mechanical Engineers, pp. 257–269, 2005.
Shi, Y., P. Lawford, and R. Hose. Review of zero-D and 1-D models of blood flow in the cardiovascular system. Biomed. Eng. Online 10:33, 2011.
Stevens, S. A., W. D. Lakin, and P. L. Penar. Modeling steady-state intracranial pressures in supine, head-down tilt and microgravity conditions. Aviation Space Environ. Med. 76:329–338, 2005.
Tiago, J., T. Guerra, and A. Sequeira. A velocity tracking approach for the data assimilation problem in blood flow simulations. Int. J. Numer. Methods Biomed. Eng. 33:e2856, 2017.
Ursino, M. and M. Giannessi. A model of cerebrovascular reactivity including the circle of Willis and cortical anastomoses. Ann. Biomed. Eng. 38:955–974, 2010.
Ursino, M. and C. A. Lodi. Interaction among autoregulation, CO2 reactivity, and intracranial pressure: a mathematical model. Am. J. Physiol.-Heart Circ. Physiol. 274:H1715–H1728, 1998.
Wakeland, W. and B. Goldstein. A review of physiological simulation models of intracranial pressure dynamics. Comput. Biol. Med. 38:1024–1041, 2008.
Xu, P., M. Kasprowicz, M. Bergsneider, and X. Hu. Improved noninvasive intracranial pressure assessment with nonlinear kernel regression. IEEE Trans. Inf. Technol. Biomed. 14:971–978, 2010.
Zhang, X., J. E. Medow, B. J. Iskandar, F. Wang, M. Shokoueinejad, J. Koueik, and J. G. Webster. Invasive and noninvasive means of measuring intracranial pressure: a review. Physiol. Meas. 38:R143, 2017.
Acknowledgments
JXW would like to thank J. Pyne and J. Wu for helpful discussions. The authors also thank the anonymous reviewers for their comments and suggestions, which helped improve the quality and clarity of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Karol Miller oversaw the review of this article.
Appendix A Algorithm: Regularizing Iterative Ensemble Kalman Method
Appendix A Algorithm: Regularizing Iterative Ensemble Kalman Method
Prior sampling: Use Latin hypercube sampling method to generate the prior state ensemble \(\{{\mathbf {x}}_j^{(0)}\}_{j=1}^{N_s}\), where \({\mathbf {x}}_j\) is jth sample of the augmented state, including major arterials’ CBFV and ABP, ICP, and unknown parameters. Let \(\rho \in (0, 1)\) and \(\tau = 1/\rho\).
For \(n = 1 : n_{max}\)
-
1.
Forward prediction:
-
(a)
Evaluate the forward intracranial model with the initial physical state, boundary conditions, and model parameters, which are updated in the last iteration. Namely, the analyzed state \({\hat{\mathbf {x}}}_j^{(n)}\) at iteration step n is propagated through the forward model \({\mathcal {F}}\) at \((n+1)\)th iteration,
$$\begin{aligned} {\mathbf {x}}_j^{(n+1)} = {\mathcal {F}}({\hat{\mathbf {x}}}_j^{n}). \end{aligned}$$(14) -
(b)
Obtain the perturbed ensemble of observation data \(\{{\mathbf {y}}_j^{(0)}\}_{j=1}^{N_s}\) based on the data uncertainty level \(\sigma _d\).
-
(c)
Calculate statistical information of predicted state and observation data. We first calculate the sample means of state and data as,
$$\begin{aligned} {\bar{\mathbf {x}}}^{(n+1)}= & {} \frac{1}{N_s}\sum _{j=1}^{N_s}{\mathbf {x}}^{(n+1)}_j \end{aligned}$$(15)$$\begin{aligned} {\bar{\mathbf {y}}}^{(n+1)}= & {} \frac{1}{N_s}\sum _{j=1}^{N_s}{\mathbf {y}}^{(n+1)}_j. \end{aligned}$$(16)The error covariances of the predicted state and observation data can then be obtained,
$$\begin{aligned} P_m^{(n+1)}= & {} \frac{1}{N_s - 1}\sum _{j=1}^{N} \bigg ({\mathbf {x}}^{(n+1)}_j - {\bar{\mathbf {x}}}) ({\mathbf {x}}^{(n+1)}_j - {\bar{\mathbf {x}}}^{(n+1)} \bigg )^T \end{aligned}$$(17)$$\begin{aligned} P_d^{(n+1)}= & {} \frac{1}{N_s - 1}\sum _{j=1}^{N}({\mathbf {y}}^{(n+1)}_j - {\bar{\mathbf {y}}}) \bigg ({\mathbf {y}}^{(n+1)}_j - {\bar{\mathbf {y}}}^{(n+1)} \bigg )^T \end{aligned}$$(18)
-
(a)
-
2.
Regularizing Bayesian analysis:
(a) Calculate the control variable \(\alpha _i^{(n+1)}\) by following sequence,
$$\begin{aligned} \alpha _i^{(n+1)} = 2^{i+1}\alpha _0, \end{aligned}$$(19)where an initial guess of \(\alpha _0 = 1\) is used in this work. The \(\alpha ^{n+1}\) is obtained as \(\alpha ^{(n+1)} \equiv \alpha ^{(n+1)}_{N}\), where N is the first integer when the inequality defined by Eq. (13) is satisfied. (b) Compute regularized Kalman gain matrix as,
$$\begin{aligned} K^{(n+1)} = P_m^{(n+1)}H^T \bigg (HP_m^{(n+1)}H^T + \alpha ^{(n+1)}P_d^{(n+1)} \bigg )^{-1}, \end{aligned}$$(20)(c) Update each state sample as follows,
$$\begin{aligned} {\hat{\mathbf {x}}}^{(n+1)} _j= {\mathbf {x}}^{(n+1)} _j + K^{(n+1)} \bigg ({\bar{\mathbf {y}}}^{(n+1)} - H{\mathbf {x}}^{(n+1)} _j \bigg ), \end{aligned}$$(21) -
3.
Stopping criteria:
If
$$\begin{aligned} \bigg |\bigg|{P_d^{(n+1)}}^{-1/2}({\mathbf {y}}^{(n+1)} - H{\bar{\mathbf {x}}}^{(n+1)}) \bigg |\bigg| \le \tau \sigma _d, \end{aligned}$$(22)then, stop the iteration. \(\sigma _d\) represents noise level of observation data.
Rights and permissions
About this article
Cite this article
Wang, JX., Hu, X. & Shadden, S.C. Data-Augmented Modeling of Intracranial Pressure. Ann Biomed Eng 47, 714–730 (2019). https://doi.org/10.1007/s10439-018-02191-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-02191-z