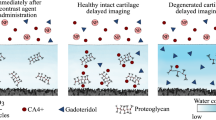
Abstract
Cartilage injuries may be detected using contrast-enhanced computed tomography (CECT) by observing variations in distribution of anionic contrast agent within cartilage. Currently, clinical CECT enables detection of injuries and related post-traumatic degeneration based on two subsequent CT scans. The first scan allows segmentation of articular surfaces and lesions while the latter scan allows evaluation of tissue properties. Segmentation of articular surfaces from the latter scan is difficult since the contrast agent diffusion diminishes the image contrast at surfaces. We hypothesize that this can be overcome by mixing anionic contrast agent (ioxaglate) with bismuth oxide nanoparticles (BINPs) too large to diffuse into cartilage, inducing a high contrast at the surfaces. Here, a dual contrast method employing this mixture is evaluated by determining the depth-wise X-ray attenuation profiles in intact, enzymatically degraded, and mechanically injured osteochondral samples (n = 3 × 10) using a microCT immediately and at 45 min after immersion in contrast agent. BiNPs were unable to diffuse into cartilage, producing high contrast at articular surfaces. Ioxaglate enabled the detection of enzymatic and mechanical degeneration. In conclusion, the dual contrast method allowed detection of injuries and degeneration simultaneously with accurate cartilage segmentation using a single scan conducted at 45 min after contrast agent administration.




Similar content being viewed by others
References
Anderson, D. D., S. Chubinskaya, F. Guilak, J. A. Martin, T. R. Oegema, S. A. Olson, and J. A. Buckwalter. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J. Orthop. Res. 29:802–809, 2011.
Bansal, P. N., N. S. Joshi, V. Entezari, M. W. Grinstaff, and B. D. Snyder. Contrast Enhanced Computed Tomography can predict the glycosaminoglycan content and biomechanical properties of articular cartilage. Osteoarthr. Cartil. 18:184–191, 2010.
Bookstein, F. L. Random walk and the existence of evolutionary rates. Paleobiology 13:446–464, 1987.
Braun, H. J., and G. E. Gold. Diagnosis of osteoarthritis: imaging. Bone 51:278–288, 2012.
Brown, T. D., R. C. Johnston, C. L. Saltzman, J. L. Marsh, and J. A. Buckwalter. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 20:739–744, 2006.
Buckwalter, J. A. Mechanical injuries of articular cartilage. Iowa Orthop. J. 12:50, 1992.
De La Vega, J. C., and U. O. Häfeli. Utilization of nanoparticles as X-ray contrast agents for diagnostic imaging applications. Contrast Media Mol. Imaging 10:81–95, 2015.
Ewers, B., V. Jayaraman, R. Banglmaier, and R. C. Haut. Rate of blunt impact loading affects changes in retropatellar cartilage and underlying bone in the rabbit patella. J. Biomech. 35:747–755, 2002.
Harris, E. D., H. G. Parker, E. L. Radin, and S. M. Krane. Effects of proteolytic enzymes on structural and mechanical properties of cartilage. Arthritis Rheumatol. 15:497–503, 1972.
Javier, D. J., N. Nitin, M. Levy, A. Ellington, and R. Richards-Kortum. Aptamer-targeted gold nanoparticles as molecular-specific contrast agents for reflectance imaging. Bioconjugate Chem. 2008. doi:10.1021/BC8001248.
Jokerst, J., V. T. Lobovkina, R. N. Zare, and S. S. Gambhir. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6:715–728, 2011.
Kallioniemi, A. S., J. S. Jurvelin, M. T. Nieminen, M. J. Lammi, and J. Töyräs. Contrast agent enhanced pQCT of articular cartilage. Phys. Med. Biol. 52:1209–1219, 2007.
Karlsson, H. L., J. Gustafsson, P. Cronholm, and L. Möller. Size-dependent toxicity of metal oxide particles—a comparison between nano- and micrometer size. Toxicol. Lett. 188:112–118, 2009.
Kokkonen, H. T., A. S. Aula, H. Kröger, J.-S. Suomalainen, E. Lammentausta, E. Mervaala, J. S. Jurvelin, and J. Töyräs. Delayed computed tomography arthrography of human knee cartilage in vivo. Cartilage 3:334–341, 2012.
Kokkonen, H. T., J. S. Jurvelin, V. Tiitu, and J. Töyräs. Detection of mechanical injury of articular cartilage using contrast enhanced computed tomography. Osteoarthr. Cartil. 19:295–301, 2011.
Kokkonen, H. T., J. Mäkelä, K. A. M. Kulmala, L. Rieppo, J. S. Jurvelin, V. Tiitu, H. M. Karjalainen, R. K. Korhonen, V. Kovanen, and J. Töyräs. Computed tomography detects changes in contrast agent diffusion after collagen cross-linking typical to natural aging of articular cartilage. Osteoarthr. Cartil. 19:1190–1198, 2011.
Kokkonen, H. T., J.-S. Suomalainen, A. Joukainen, H. Kröger, J. Sirola, J. S. Jurvelin, J. Salo, and J. Töyräs. In vivo diagnostics of human knee cartilage lesions using delayed CBCT arthrography. J. Orthop. Res. 32:403–412, 2014.
Lin, P. M., C.-T. C. Chen, and P. A. Torzilli. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthr. Cartil. 12:485–496, 2004.
Liu, X. H., L. Zhong, S. Huang, S. X. Mao, T. Zhu, and J. Y. Huang. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 6:1522–1531, 2012.
Mohan, R. S. In your element: green bismuth. Nat. Chem. 2:336, 2010.
Moody, H. R., C. P. Brown, J. C. Bowden, R. W. Crawford, D. L. S. McElwain, and A. O. Oloyede. In vitro degradation of articular cartilage: does trypsin treatment produce consistent results? J. Anat. 209:259–267, 2006.
Myller, K. A. H., M. J. Turunen, J. T. J. Honkanen, S. P. Väänänen, J. T. Iivarinen, J. Salo, J. S. Jurvelin, and J. Töyräs. In vivo contrast-enhanced cone beam CT provides quantitative information on articular cartilage and subchondral bone. Ann. Biomed. Eng. 45:811–818, 2017.
Olson, S. A., B. D. Furman, V. B. Kraus, J. L. Huebner, and F. Guilak. Therapeutic opportunities to prevent post-traumatic arthritis: lessons from the natural history of arthritis after articular fracture. J. Orthop. Res. 33:1266–1277, 2015.
Pan, D., E. Roessl, J.-P. Schlomka, S. D. Caruthers, A. Senpan, M. J. Scott, J. S. Allen, H. Zhang, G. Hu, P. J. Gaffney, E. T. Choi, V. Rasche, S. A. Wickline, R. Proksa, and G. M. Lanza. Computed tomography in color: NanoK-enhanced spectral CT molecular imaging. Angew. Chem. 122:9829–9833, 2010.
Panula, H. E., M. M. Hyttinen, J. P. Arokoski, T. K. Långsjö, A. Pelttari, I. Kiviranta, and H. J. Helminen. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann. Rheum. Dis. 57:237–245, 1998.
Peat, G., C. Bergknut, R. Frobell, A. Jöud, and M. Englund. Population-wide incidence estimates for soft tissue knee injuries presenting to healthcare in southern Sweden: data from the Skåne Healthcare Register. Arthritis Res. Ther. 16:R162, 2014.
Piscaer, T. M., J. H. Waarsing, N. Kops, P. Pavljasevic, J. A. N. Verhaar, G. J. V. M. van Osch, and H. Weinans. In vivo imaging of cartilage degeneration using μCT-arthrography. Osteoarthr. Cartil. 16:1011–1017, 2008.
Rieppo, J., J. Töyräs, M. T. Nieminen, V. Kovanen, M. M. Hyttinen, R. K. Korhonen, J. S. Jurvelin, and H. J. Helminen. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs 175:121–132, 2003.
Silvast, T. S., J. S. Jurvelin, A. S. Aula, M. J. Lammi, and J. Töyräs. Contrast agent-enhanced computed tomography of articular cartilage: association with tissue composition and properties. Acta Radiol. 50:78–85, 2009.
Silvast, T. S., H. T. Kokkonen, J. S. Jurvelin, T. M. Quinn, M. T. Nieminen, and J. Töyräs. Diffusion and near-equilibrium distribution of MRI and CT contrast agents in articular cartilage. Phys. Med. Biol. 54:6823–6836, 2009.
Stewart, R. C., P. N. Bansal, V. Entezari, H. Lusic, R. M. Nazarian, B. D. Snyder, and M. W. Grinstaff. Contrast-enhanced CT with a high-affinity cationic contrast agent for imaging ex vivo bovine, intact ex vivo rabbit, and in vivo rabbit cartilage. Radiology 266:141–150, 2013.
Stewart, R. C., J. T. J. Honkanen, H. T. Kokkonen, V. Tiitu, S. Saarakkala, A. Joukainen, B. D. Snyder, J. S. Jurvelin, M. W. Grinstaff, and J. Töyräs. Contrast-enhanced computed tomography enables quantitative evaluation of tissue properties at intrajoint regions in cadaveric knee cartilage. Cartilage 2016. doi:10.1177/1947603516665443.
Stewart, R. C., A. N. Patwa, H. Lusic, J. D. Freedman, M. Wathier, B. D. Snyder, A. Guermazi, and M. W. Grinstaff. Synthesis and preclinical characterization of a cationic iodinated imaging contrast agent (CA4+) and its use for quantitative computed tomography of ex vivo human hip cartilage. J. Med. Chem. 60:5543–5555, 2017.
Väänänen, S. P., J. S. Jurvelin, and H. Isaksson. Estimation of 3D shape, internal density and mechanics of proximal femur by combining bone mineral density images with shape and density templates. Biomech. Model. Mechanobiol. 11:791–800, 2012.
Verma, A., and F. Stellacci. Effect of surface properties on nanoparticle-cell interactions. Small 6:12–21, 2010.
Yoo, H. J., S. H. Hong, J.-Y. Choi, I. J. Lee, S. J. Kim, J.-A. Choi, and H. S. Kang. Contrast-enhanced CT of articular cartilage: experimental study for quantification of glycosaminoglycan content in articular cartilage. Radiology 261:805–812, 2011.
Zhang, X.-D., D. Wu, X. Shen, J. Chen, Y.-M. Sun, P.-X. Liu, and X.-J. Liang. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 33:6408–6419, 2012.
Zhang, X.-D., D. Wu, X. Shen, P.-X. Liu, N. Yang, B. Zhao, H. Zhang, Y.-M. Sun, L.-A. Zhang, and F.-Y. Fan. Size-dependent in vivo toxicity of peg-coated gold nanoparticles. Int. J. Nanomed. 6:2071–2081, 2011.
Acknowledgments
Academy of Finland (Projects 269315, 288531, and 288531), Kuopio University Hospital (VTR 5041746, 5203101, PY 210), Magnus Ehrnrooth Foundation, Finnish Cultural Foundation, and Doctoral Programme in Science, Technology and Computing (SCITECO) of University of Eastern Finland are acknowledged for financial support. MSc (Tech) Jaakko Sarin is acknowledged for assistance with the histological analysis.
Conflict of interest
Authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Sean S. Kohles oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Saukko, A.E.A., Honkanen, J.T.J., Xu, W. et al. Dual Contrast CT Method Enables Diagnostics of Cartilage Injuries and Degeneration Using a Single CT Image. Ann Biomed Eng 45, 2857–2866 (2017). https://doi.org/10.1007/s10439-017-1916-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1916-3