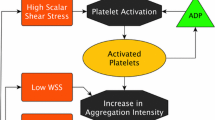
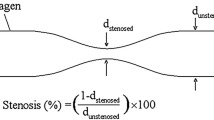
Abstract
The ability to predict the timescale of thrombotic occlusion in stenotic vessels may improve patient risk assessment for thrombotic events. In blood contacting devices, thrombosis predictions can lead to improved designs to minimize thrombotic risks. We have developed and validated a model of high shear thrombosis based on empirical correlations between thrombus growth and shear rate. A mathematical model was developed to predict the growth of thrombus based on the hemodynamic shear rate. The model predicts thrombus deposition based on initial geometric and fluid mechanic conditions, which are updated throughout the simulation to reflect the changing lumen dimensions. The model was validated by comparing predictions against actual thrombus growth in six separate in vitro experiments: stenotic glass capillary tubes (diameter = 345 µm) at three shear rates, the PFA-100® system, two microfluidic channel dimensions (heights = 300 and 82 µm), and a stenotic aortic graft (diameter = 5.5 mm). Comparison of the predicted occlusion times to experimental results shows excellent agreement. The model is also applied to a clinical angiography image to illustrate the time course of thrombosis in a stenotic carotid artery after plaque cap rupture. Our model can accurately predict thrombotic occlusion time over a wide range of hemodynamic conditions.







Similar content being viewed by others
Change history
04 November 2019
This erratum is to correct the heading of column 2 (titled ���b���) in Table��1, which was missing proper units. The heading for that column was revised to include proper units, reading ���b (�� 10���6 s)���.
04 November 2019
This erratum is to correct the heading of column 2 (titled ���b���) in Table��1, which was missing proper units. The heading for that column was revised to include proper units, reading ���b (�� 10���6 s)���.
Abbreviations
- a, c :
-
Fitting constants (µm/min)
- b, d :
-
Fitting constants (s)
- D :
-
Lumen diameter
- J :
-
Thrombus growth rate (µm/min)
- J max :
-
Maximum thrombus growth rate (µm/min)
- L :
-
Stenosis length
- r :
-
Lumen radius
- S :
-
Wall shear rate (s−1)
- \(S_{J\hbox{max} }\) :
-
Shear rate corresponding to maximum thrombus growth rate (s−1)
- t :
-
Time
- t lag :
-
Lag time
- \(t_{\text{Occ}}\) :
-
Occlusion time
- vWF:
-
von Willebrand factor
- z :
-
The axial direction
References
Antaki, J. F., M. R. Ricci, J. E. Verkaik, S. T. Snyder, T. M. Maul, et al. PediaFlow™ Maglev ventricular assist device: a prescriptive design approach. Cardiovasc. Eng. Technol. 1:104–121, 2010.
Badimon, L., J. J. Badimon, V. T. Turitto, S. Vallabhajosula, and V. Fuster. Platelet thrombus formation on collagen type I. A model of deep vessel injury. Influence of blood rheology, von Willebrand factor, and blood coagulation. Circulation 78:1431–1442, 1988.
Bark, D. L. The Hemodynamics during Thrombosis and Impact on Thrombus Growth. Atlanta, GA: Mech. Eng., Georgia Institute of Technology, 2010.
Bark, D. L., and D. N. Ku. Wall shear over high degree stenoses pertinent to atherothrombosis. J. Biomech. 43:2970–2977, 2010.
Bark, D. L., A. N. Para, and D. N. Ku. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol. Bioeng. 109:2642–2650, 2012.
Barstad, R. M., H. Roald, Y. Cui, V. Turitto, and K. Sakariassen. A perfusion chamber developed to investigate thombus formation and shear profiles in flowing native human blood at the apex of a well-defined stenosis. Atertio. Thromb. Vasc. Biol. 14:1984–1991, 1994.
Bernardo, A., A. L. Bergeron, C. W. Sun, P. Guchhait, M. A. Cruz, et al. Von Willebrand factor present in fibrillar collagen enhances platelet adhesion to collagen and collagen-induced platelet aggregation. J. Thromb. Haemost. 2:660–669, 2004.
Bland, J. M., and D. G. Altman. Statistics notes: calculating correlation coefficients with repeated observations: Part 1—correlation within subjects. BMJ 310:466, 1995.
Casa, L. D. C., D. H. Deaton, and D. N. Ku. Role of high shear rate in thrombosis. J. Vasc. Surg. 61:1068–1080, 2015.
Casa, L. D. C., and D. N. Ku. Geometric design of microfluidic chambers: platelet adhesion versus accumulation. Biomed. Microdevices 16:115–126, 2014.
Casa, L. D. C., and D. N. Ku. High shear thrombus formation under pulsatile and steady flow. Cardiovasc. Eng. Technol. 5:154–163, 2014.
Cito, S., M. D. Mazzeo, and L. Badimon. A review of macroscopic thrombus modeling methods. Thromb. Res. 131:116–124, 2013.
DuMouchel, W. H., and F. L. O’Brien, Integrating a Robust Option into a Multiple Regression Computing Environment., Computer Science and Statistics: Proceedings of the 21st Symposium on the Interface., American Statistical Association, Alexandria, VA, 1989.
Epstein, F. H., J. Lefkovits, E. F. Plow, and E. J. Topol. Mechanisms of disease: platelet glycoprotein IIb/IIIa receptors in cardiovascular medicine. New Engl. J. Med. 332:1553–1559, 1995.
Escudero, C., M. Santos, J. Buján, M. Fuente, N. G. Honduvilla, et al. Optical aggregometry versus the PFA-100: experimental studies in pigs treated with propofol. Platelets 12:133–137, 2001.
Flamm, M. H., T. V. Colace, M. S. Chatterjee, H. Jing, S. Zhou, et al. Multiscale prediction of patient-specific platelet function under flow. Blood 120:190–198, 2012.
Hellums, J. 1993 Whitaker Lecture: biorheology in thrombosis research. Ann. Biomed. Eng. 22:445–455, 1994.
Holland, P. W., and R. E. Welsch. Robust regression using iteratively reweighted least-squares. Commun. Stat. 6:813–827, 1977.
Huck, V., M. F. Schneider, C. Gorzelanny, and S. W. Schneider. The various states of von Willebrand factor and their function in physiology and pathophysiology. Thromb. Haemost. 111:598–609, 2014.
Hunter, J. V., and J. K. Chin. Carotid Angiography. In: Vascular Disease: Surgical & Interventional Therapy, edited by D. E. Strandness Jr, and Van Breda. New York: Churchill Livingstone, 1994, pp. 273–287.
Ku, D. N., and C. J. Flannery. Development of a flow-through system to create occluding thrombus. Biorheology 44:273–284, 2007.
Leuser, C., S. Schlottmann, R. Siekmann, M. Heidt, A. Moritz, et al. Use of the platelet function analyser PFA-100™ in juvenile pigs. Comp. Clin. Pathol. 21:761–767, 2011.
Li, M. Microfluidic System for Thrombosis Under Multiple Shear Rates and Platelet Therapies. Atlanta, GA: Biomed. Eng, Georgia Institute of Technology, 2013.
Li, M., N. A. Hotaling, D. N. Ku, and C. R. Forest. Microfluidic thrombosis under multiple shear rates and antiplatelet therapy doses. PLoS One 9:e82493, 2014.
Li, M., D. Ku, and C. Forest. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 12:1355–1362, 2012.
Nesbitt, W. S., E. Westein, F. J. Tovar-Lopez, E. Tolouei, A. Mitchell, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 15:665–673, 2009.
Nobili, M., J. Sheriff, U. Morbiducci, A. Redaelli, and D. Bluestein. Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 54:64–72, 2008.
Para, A. N., and D. N. Ku. A low-volume, single pass in vitro system of high shear thrombosis in a stenosis. Thromb. Res. 131:418–424, 2013.
Reneman, R. S., T. Arts, and A. P. G. Hoeks. Wall shear stress—an important determinant of endothelial cell function and structure—in the arterial system in vivo. J. Vasc. Res. 43:251–269, 2006.
Rowley, J. W., A. V. Finn, P. A. French, L. K. Jennings, D. Bluestein, et al. Cardiovascular devices and platelet interactions: understanding the role of injury, flow, and cellular responses. Circ. Cardiovasc. Interv. 5:296–304, 2010.
Ruggeri, Z. M. Von Willebrand factor: looking back and looking forward. Thromb. Haemost. 98:55–62, 2007.
Savage, B., E. Saldivar, and Z. M. Ruggeri. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell 84:289–297, 1996.
Siemens, PFA-100® System: Getting Started Guide, 2008.
Strandness, D. E., and A. van Breda. Vascular Diseases: Surgical and Interventional Therapy. London: Churchill Livingstone, 1993.
Strony, J., A. Beaudoin, D. Brands, and B. Adelman. Analysis of shear stress and hemodynamic factors in a model of coronary artery stenosis and thrombosis. Am. J. Physiol. 265:H1787–H1796, 1993.
Tomaiuolo, M., T. J. Stalker, J. D. Welsh, S. L. Diamond, T. Sinno, et al. A systems approach to hemostasis: 2. Computational analysis of molecular transport in the thrombus microenvironment. Blood 124:1816–1824, 2014.
Wang, W., and M. R. King. Multiscale modeling of platelet adhesion and thrombus growth. Ann. Biomed. Eng. 40:2345–2354, 2012.
Xu, Z., M. Kamocka, M. Alber, and E. D. Rosen. Computational approaches to studying thrombus development. Atertio. Thromb. Vasc. Biol. 31:500–505, 2011.
Xu, Z., J. Lioi, J. Mu, M. M. Kamocka, X. Liu, et al. A multiscale model of venous thrombus formation with surface-mediated control of blood coagulation cascade. Biophys. J. 98:1723–1732, 2010.
Yun, B., J. Wu, H. Simon, S. SArgunon, F. Sotiropoulos, et al. A numerical investigation of blood damage in the hinge area of aortic bileaflet mechanical heart valves during the leakage phase. Ann. Biomed. Eng. 40:1468–1485, 2012.
Zydney, A., and C. Colton. Augmented solute transport in the shear flow of a concentrated suspension. Physicochem. Hydrodyn. 10:77–96, 1988.
Acknowledgments
The authors gratefully acknowledge the assistance of and Peter Costandi (Endologix, Inc.) and Susan Hastings in conducting the aortic graft experiments. This work was supported by a grant from the Center for Pediatric Innovation, Georgia Institute of Technology, Children’s Healthcare of Atlanta, and Emory University; Lawrence P. Huang Chair Funds, Georgia Institute of Technology; and the John and Mary Brock Discovery Fund. LDCC was supported by an American Heart Association Pre-Doctoral Fellowship (14PRE18080005). Aortic graft experiments were funded by Endologix, Inc. DNK and LDCC acted as paid consultants for Endologix, Inc. We declare that we have no other conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Marmar Mehrabadi and Lauren D. C. Casa contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mehrabadi, M., Casa, L.D.C., Aidun, C.K. et al. A Predictive Model of High Shear Thrombus Growth. Ann Biomed Eng 44, 2339–2350 (2016). https://doi.org/10.1007/s10439-016-1550-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1550-5