Abstract
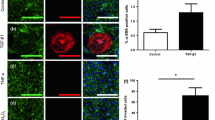
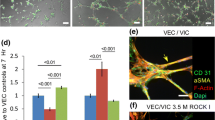
Lack of understanding of the early mechanisms of aortic valve stenosis and calcification hinders the development of diagnostic and therapeutic intervention strategies. Inflammation is a known component of early aortic valve disease and can induce mesenchymal transformation in a subset of aortic valve endothelial cells. Here we present a three-dimensional culture system that allows transforming and non-transforming cells to be independently isolated and analyzed. We have used the system to identify and characterize the dynamic invasion and phenotypic transition of two distinct subsets of endothelial cells: those that invade and transform under TNFα treatment, and those that resist mesenchymal transformation and remain endothelial. We determine that non-transformed cells maintain control levels of endothelial genes VE-cadherin and eNOS, while transformed cells lose these endothelial characteristics and upregulate α-smooth muscle actin. Both subsets of cells have an inflammatory phenotype marked by increased ICAM-1, but transformed cells have increased MMP-9, Notch1, TGF-β, and BMP-4, while non-transformed cells do not. Transformed cells also have distinct effects on alignment of collagen fibers as they invade the hydrogel system, which is not found in control endothelial or interstitial valve cells. Understanding the role of transforming and non-transforming endothelial cells in valve disease will provide an important pathological link between early inflammation and later stages of disease. Discovery of the molecular signature of transformation-resistant endothelial cells could inform development of treatment strategies that promote survival of the valve endothelium.







Similar content being viewed by others
References
Aikawa, E., M. Nahrendorf, D. Sosnovik, V. M. Lok, F. A. Jaffer, M. Aikawa, and R. Weissleder. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115:377–386, 2007.
Ankeny, R. F., V. H. Thourani, D. Weiss, J. D. Vega, W. R. Taylor, R. M. Nerem, and H. Jo. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves: association with low BMP antagonists and SMAD6. PLoS ONE 6:e20969, 2011.
Balachandran, K., P. Alford, J. Wylie-Sears, J. A. Goss, A. Grosberg, J. Bischoff, E. Aikawa, R. A. Levine, and K. K. Parker. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc. Natl. Acad. Sci. U.S.A. 108:19943–19948, 2011.
Bischoff, J., and E. Aikawa. Progenitor cells confer plasticity to cardiac valve endothelium. J. Cardiovasc. Transl. Res. 4:710–719, 2011.
Boström, K., K. E. Watson, S. Horn, C. Wortham, I. M. Herman, and L. L. Demer. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Invest. 91:1800–1809, 1993.
Butcher, J. T., and R. M. Nerem. Valvular endothelial cells regulate the phenotype of interstitial cells in co-culture: effects of steady shear stress. Tissue Eng. 12:905–915, 2006.
Cano, A., M. A. Pérez-Moreno, I. Rodrigo, A. Locascio, M. J. Blanco, M. G. del Barrio, F. Portillo, and M. A. Nieto. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2:76–83, 2000.
Chang, A. C. Y., Y. Fu, V. C. Garside, K. Niessen, L. Chang, M. Fuller, A. Setiadi, J. Smrz, A. Kyle, A. Minchinton, M. Marra, P. A. Hoodless, and A. Karsan. Notch initiates the endothelial-to-mesenchymal transition in the atrioventricular canal through autocrine activation of soluble guanylyl cyclase. Dev. Cell 21:288–300, 2011.
Edep, M. E., J. Shirani, P. Wolf, and D. L. Brown. Matrix metalloproteinase expression in nonrheumatic aortic stenosis. Cardiovasc. Pathol. 9:281–286, 2000.
Fondard, O., D. Detaint, B. Lung, C. Choqueux, H. Adle-Biassette, M. Jarraya, U. Hvass, J. P. Couetil, D. Henin, J. B. Michel, A. Vahanian, and M. P. Jacob. Extracellular matrix remodeling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 26:1333–1341, 2005.
Galichon, P., and A. Hertig. Epithelial to mesenchymal transition as a biomarker in renal fibrosis: are we ready for the bedside? Fibrogenesis Tissue Repair 4:11, 2011.
Garg, V., A. N. Muth, J. F. Ransom, M. K. Schluterman, R. Barnes, I. N. King, P. D. Grossfeld, and D. Srivastava. Mutations in NOTCH1 cause aortic valve disease. Nature 437:270–274, 2005.
Ghaisas, N., J. Foley, D. O’Briain, P. Crean, D. Kelleher, and M. Walsh. Adhesion molecules in nonrheumatic aortic valve disease: endothelial expression, serum levels and effects of valve replacement. J. Am. Coll. Cardiol. 36:2257–2262, 2000.
Go, A. S., et al. Heart disease and stroke statistics—2013 Update: a report from the American Heart Association. Circulation 127:e6–e245, 2013.
Goldbarg, S. H., S. Elmariah, M. A. Miller, and V. Fuster. Insights into degenerative aortic valve disease. J. Am. Coll. Cardiol. 50:1205–1230, 2007.
Gould, R. A., and J. T. Butcher. Isolation of valvular endothelial cells. J. Vis. Exp. 46:1–5, 2010.
Guerraty, M. A., G. R. Grant, J. W. Karanian, O. A. Chiesa, W. F. Pritchard, and P. F. Davies. Hypercholesterolemia induces side-specific phenotypic changes and peroxisome proliferator-activated receptor-pathway activation in swine aortic valve endothelium. Arterioscler. Thromb. Vasc. Biol. 30:225–231, 2010.
Hjortnaes, J., J. Butcher, J. L. Figueiredo, M. Riccio, R. H. Kohler, K. M. Kozloff, R. Weissleder, and E. Aikawa. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. Eur. Heart J. 31:1975–1984, 2010.
Hollier, B. G., A. A. Tinnirello, S. J. Werden, K. W. Evans, J. H. Taube, T. R. Sarkar, N. Sphyris, M. Shariati, S. V. Kumar, V. L. Battula, J. I. Herschkowitz, R. Guerra, J. T. Chang, N. Miura, J. M. Rosen, and S. A. Mani. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 73:1981–1992, 2013.
Jia, Q., B. W. McDill, S.-Z. Li, C. Deng, C.-P. Chang, and F. Chen. Smad signaling in the neural crest regulates cardiac outflow tract remodeling through cell autonomous and non-cell autonomous effects. Dev. Biol. 311:172–184, 2007.
Jian, B., N. Narula, Q. Li, E. Mohler, and R. Levy. Progression of aortic valve stenosis: TGF-beta 1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 75:457–465, 2003.
Kalluri, R., and R. A. Weinberg. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119:1420–1428, 2009.
Liu, A. C., and A. I. Gotlieb. Transforming growth factor-β regulates in vitro heart valve repair by activated valve interstitial cells. Am. J. Pathol. 173:1275–1285, 2008.
Mahler, G. J., E. J. Farrar, and J. T. Butcher. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler. Thromb. Vasc. Biol. 33:121–130, 2013.
Mohler, E. R., M. K. Chawla, A. W. Chang, N. Vyavahare, R. J. Levy, L. Graham, and F. H. Gannon. Identification and characterization of calcifying valve cells from human and canine aortic valves. J. Heart Valve Dis. 8:254–260, 1999.
Paranya, G., S. Vineberg, E. Dvorin, S. Kaushal, S. J. Roth, E. Rabkin, F. J. Schoen, and J. Bischoff. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am. J. Pathol. 159:1335–1343, 2001.
Richards, J., I. El-Hamamsy, S. Chen, Z. Sarang, P. Sarathchandra, M. H. Yacoub, A. H. Chester, and J. T. Butcher. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am. J. Pathol. 182:1922–1931, 2013.
Simmons, C. A., G. R. Grant, E. Manduchi, and P. F. Davies. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ. Res. 96:792–799, 2005.
Stewart, W. J., and B. A. Carabello. Aortic valve disease. In: Textbook of Cardiovascular Medicine, edited by E. J. Topol, R. M. Califf, E. N. Prystowsky, J. D. Thomas, and P. D. Thompson. Philadelphia, PA: Lippincott Williams & Wilkins, 2006, pp. 366–388.
Sucosky, P., K. Balachandran, A. Elhammali, H. Jo, and A. P. Yoganathan. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-β1-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 29:254–260, 2009.
Walker, G. A., K. S. Masters, D. N. Shah, K. S. Anseth, and L. A. Leinwand. Valvular myofibroblast activation by transforming growth factor-beta: implications for pathological extracellular matrix remodeling in heart valve disease. Circ. Res. 95:253–260, 2004.
Wylie-Sears, J., E. Aikawa, R. A. Levine, J.-H. Yang, and J. Bischoff. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler. Thromb. Vasc. Biol. 31:598–607, 2011.
Yang, J.-H., J. Wylie-Sears, and J. Bischoff. Opposing actions of Notch1 and VEGF in post-natal cardiac valve endothelial cells. Biochem. Biophys. Res. Commun. 374:512–516, 2008.
Acknowledgments
The authors would like to thank Shirks Meats of Dundee, NY for providing porcine aortic valves. This study was supported by the National Science Foundation Graduate Research Fellowship (EF), the Alfred P. Sloan Foundation (EF), NIH Grant HL110328, NSF CBET-0955172, and the LeDucq Foundation.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Sriram Neelamegham oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Farrar, E.J., Butcher, J.T. Heterogeneous Susceptibility of Valve Endothelial Cells to Mesenchymal Transformation in Response to TNFα. Ann Biomed Eng 42, 149–161 (2014). https://doi.org/10.1007/s10439-013-0894-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-013-0894-3