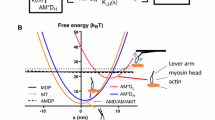
Abstract
We employ a mechanical model of sarcomere to quantitatively investigate how adenosine triphosphate (ATP) concentration affects motor force regulation during skeletal muscle contraction. Our simulation indicates that there can be negative cross-bridges resisting contraction within the sarcomere and higher ATP concentration would decrease the resistance force from negative cross-bridges by promoting their timely detachment. It is revealed that the motor force is well regulated only when ATP concentration is above a certain level. These predictions may provide insights into the role of ATP in regulating coordination among multiple motors.





Similar content being viewed by others
References
Schliwa, M., Woehlke, G.: Molecular motors. Nature 422, 759–765 (2003)
Guérin, T., Prost, J., Martin, P., et al.: Coordination and collective properties of molecular motors: theory. Curr. Opin. Cell Biol. 22, 14–20 (2010)
Mao, H.Z., Saha, M., Reyes-Aldrete, E., et al.: Structural and molecular basis for coordination in a viral DNA packaging motor. Cell Rep. 14, 2017–2029 (2016)
Tanner, B.C., Daniel, T.L., Regnier, M.: Sarcomere lattice geometry influences cooperative myosin binding in muscle. PLoS Comput. Biol. 3, e115 (2016)
Piazzesi, G., Reconditi, M., Linari, M., et al.: Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131, 784–795 (2007)
Chen, B., Gao, H.: Motor force homeostasis in skeletal muscle contraction. Biophys. J. 101, 396–403 (2011)
Dong, C., Chen, B.: Catch-slip bonds can be dispensable for motor force regulation during skeletal muscle contraction. Phys. Rev. E. 92, 012723 (2015)
Chen, B.: Self-regulation of motor force through chemomechanical coupling in skeletal muscle contraction. J. Appl. Mech. 80, 857–865 (2013)
Siemankowski, R.F., Wiseman, M.O., White, H.D.: ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc. Natl. Acad. Sci. USA 82, 658–662 (1985)
Cooke, R., Bialek, W.: Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys. J. 28, 241 (1979)
Stienen, G.J., Laarse, W.J.V.D., Elzinga, G.: Dependency of the force–velocity relationships on Mg ATP in different types of muscle fibers from Xenopus laevis. Biophys. J. 53, 849–855 (1988)
Ferenczi, M.A., Goldman, Y.E., Simmons, R.M.: The dependence of force and shortening velocity on substrate concentration in skinned muscle fibres from Rana temporaria. J. Physiol. (Oxford, U.K.) 350, 519–543 (1984)
Erdmann, T., Schwarz, U.S.: Bistability of cell-matrix adhesions resulting from nonlinear receptor-ligand dynamics. Biophys. J. 91, L60–L62 (2006)
Erdmann, T., Schwarz, U.S.: Impact of receptor-ligand distance on adhesion cluster stability. Eur. Phys. J. E 22, 123–137 (2007)
Piazzesi, G., Lombardi, V.: A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys. J. 68, 1966–1979 (1995)
Duke, T.: Molecular model of muscle contraction. Proc. Natl. Acad. Sci. USA 96, 2770–2775 (1999)
Xie, X.S.: Enzyme kinetics, past and present. Science 342, 1457–1459 (2013)
Schoenberg, M.: Characterization of the myosin adenosine triphosphate (M.ATP) crossbridge in rabbit and frog skeletal muscle fibers. Biophys. J. 54, 135–148 (1988)
Gillespie, D.T.: Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 81, 2340–2361 (1977)
Gillespie, D.T.: A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 22, 403–434 (1976)
Hill, A.: The heat of shortening and the dynamic constants of muscle. Proc. R. Soc. Lond. Ser. B. 126, 136–195 (1938)
Howard, J.: Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates, Sunderland (2001)
Huxley, A.F., Simmons, R.M.: Mechanical properties of the cross-bridges of frog striated muscle. J. Physiol. (Oxford, U.K.) 218 Suppl, 59–60 (1971)
Morgan, K.G., Gangopadhyay, S.S.: Invited review: cross-bridge regulation by thin filament-associated proteins. J. Appl. Physiol. 91, 953–962 (2001)
Acknowledgements
The project was supported by the National Natural Science Foundation of China (Grants 11372279, 11572285).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, J., Dong, C. & Chen, B. Effects of adenosine triphosphate concentration on motor force regulation during skeletal muscle contraction. Acta Mech. Sin. 33, 243–249 (2017). https://doi.org/10.1007/s10409-017-0637-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-017-0637-z