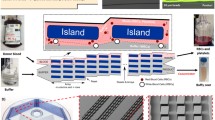
Abstract
In this article, we demonstrate for the first time the integration of a micromixer unit for the creation of a cancer cell–microbead complex, and an inertial flow unit for the detection and separation in a centrifugal platform. The two units work under different operational principles but both exploit the centrifugal pseudo-force. The units achieve a high level of binding efficiency and a mechanism for cell sorting and guiding with the established asymmetric inertial flow system, respectively. The design of the passive micromixer takes advantage of the centrifugal force in an orthogonal direction to create what has been termed “flipping” to increase chaotic advection in the unit by turning the microchannel contents 180° at each turn. Blood was spiked into the system to identify maximum operational range. In non-spiked samples, cancer cells (MCF7) and microbeads bind together to generate cell–bead complexes (MCF7-PS) with a binding efficiency of 97.1 %; however, blood-spiked samples of 2 % v/v blood content were found to have a separation of 92.5 %, which diminished further with increasing blood content (5 % v/v blood). Once the complexes enter the inertial flow unit under these conditions, it remains in high operational flow-focusing standard with up to 98.7 % ± 1.4 of the introduced cancer cells reaching the designated outlet; for both units, unpaired statistical t tests show P < 5 with 95 % confidence level. This integration allows for the positive detection of cancer cells with reactive epitopes while the increased complex averaged size of cancer cell–microbeads standardizes the flow rate required for size-based flow-focusing. It can also be optimized for negative selection or multivariate detection of different cell biomarkers by enhancing sedimentation forces.









Similar content being viewed by others
References
Abaxis.com. Accessed May, 2013
Asmolov E (1999) The inertial lift on a spherical particle in a plane Poiseuille flow at large channel Reynolds number. J Fluid Mech 381:63–87
Boubnov BM, Golitsyn GS (1995).Convection in rotating fluids. Springer, Berlin, p 8. ISBN: 0-7923-3371-3
Brenner T, Glatze T, Zengerle R, Ducree J (2005) Batch-mode mixing on centrifugal microfluidic platforms. Lab Chip 5:146–150
Burger R, Ducree J (2012) Handling and analysis of cells and bioparticles on centrifugal microfluidic platforms. Expert Rev Mol Diagn 12:407–421
Capretto L, Cheng W, Hill M, Zhang X (2011) Micromixing within microfluidic devices. Top Curr Chem 304:27–68
Chen G, Albertsa C, Rodriguez W, Toner M (2010) Concentration and purification of human immunodeficiency virus type 1 virions by microfluidic separation of superparamagnetic nanoparticles. Anal Chem 82:723. doi:10.1021/ac9024522
Cho YK, Lee JG, Park JM, Lee BS (2007) One-step pathogen specific DNA extraction from whole blood on a centrifugal microfluidic device. Lab Chip 7:565–573
Fang W, Yang J (2009) A novel microreactor with 3D rotating flow to boost fluid reaction and mixing of viscous fluids. Sens Actuators B Chem 140:629–642
Godino N, Gorkin R III, Linares AV, Burger R, Ducree J (2013) Comprehensive integration of homogeneous bioassays via centrifugo-pneumatic cascading. Lab Chip 13:685
Gossett D, Di Carlo D (2009) Particle focusing mechanisms in curving confined flows. Anal Chem 81:8459–8465
Hansson J, Karlsson J, Haraldsson T, Brismar H, van der Wijngaart W, Russom A (2012) Inertial microfluidics in parallel channels for high-throughput applications. Lab Chip 12:4644–4650
Hogg AJ (1994) Inertial migration of a non-neutrally buoyant particle in a two-dimensional shear flow. J Fluid Mech 272:285–318
Hong CC, Choi J-W, Ahn CH (2004) A novel in-plane passive microfluidic mixer with modified Tesla structures. Lab Chip 4:109–113. doi:10.1039/B305892A
Hou HW, Bhagat AAS, Lee WC, Huang S, Han J, Lim CT (2011) Microfluidic devices for blood fractionation. Micromachines 2:319–343. doi:10.3390/mi2030319
Humphry K, Kulkarni P, Weitz D, Morris J, Stone J (2010) Axial and lateral particle ordering in finite Reynolds number channel flows. Phys Fluids 22:081703
Joseph DD (2002) Power law correlations for the lift force on a particle in plane Poiseuille flow DDJ/2002/papers/Wang-PLCorr/nt_lift.doc
Jung JH, Kim GY, Seo TS (2011) An integrated passive micromixer–magnetic separation–capillary electrophoresis microdevice for rapid and multiplex pathogen detection at the singlecell level. Lab Chip 11(20):3465–3470. doi:10.1039/c1lc20350a
Kamholz A, Weigl B, Finlayson B, Yager P (1999) Quantitative analysis of molecular interaction in a microfluidic channel: T-sensor. Anal Chem 71:5340–5347
Kirby D, Siegrist J, Kijanka G, Burger R, Sheils O, O’Leary J, Ducrée J (2012) Centrifugo-magnetophoretic particle separation. Microfluid Nanofluid. doi:10.1007/s10404-012-1007-6
Kitsara M, Aguirre G, Efremov V, Ducree J (2013) Lab-on-a-disc platform for particle focusing induced by inertial forces. In: Proceedings of SPIE 8765, Bio-MEMS and Medical Microdevices 87650R. doi:10.1117/12.2017438
Kuntaegowdanahalli S, Bhagat S, Kumarb G, Papautsky I (2009) Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 9:2973–2980
La M, Park SJ, Kim HW, Park JJ, Ahn KT, Ryew SM, Kim DS (2013) A centrifugal force-based serpentine micromixer (CSM) on a plastic lab-on-a-disk for biochemical assays. Microfluid Nanofluid 15:87–98. doi:10.1007/s10404-012-1127-z
Levin S, Giddings J (1991) Continuous separation of particles from macromolecules in split-flow thin (SPLITT) cells. J Chem Tech Biotechnol 50:43–56
Lu L, Ryu K, Liu C (2002) A magnetic microstirrer and array for microfluidic mixing. J Microelectromech Syst 11:462–469
Matas J, Morris J, Guazelli E (2009) Lateral force on a rigid sphere in large-inertia laminar pipe flow. J Fluid Mech 621:59–67
Morijiri T, Hikida T, Yamada M, Seki M (2010) Microfluidic counterflow centrifugal elutriation system for sedimentation-based cell separation. 978-0-9798064-3-8/μTAS 2010/$20©2010 CBMS
Morijiri T, Sunahiro S, Senaha M, Yamada M, Seki M (2011) Sedimentation pinched-flow fractionation for size- and density-based particle sorting in microchannels. Microfluid Nanofluid 11:105–110
Mugele F, Baret JC (2005) Electrowetting: from basics to applications. J Phys Condens Matter 17:R705–R774
Noroozi Z, Kido H, Micic M, Pan H, Bartolome C, Princevac M, Zovaland J, Madou M (2009) Reciprocating flow-based centrifugal microfluidics mixer. Rev Sci Instrum 80:075102
Oakey J, Applegate R Jr, Arellano E, Carlo DD, Graves S, Toner M (2010) Particle focusing in staged inertial microfluidic devices for flow cytometry. Anal Chem 82:3862–3867
Pappas D, Wong K (2007) Cellular separations: a review of new challenges in analytical chemistry. Anal Chim Acta 601:26–35
Paterlini-Brechot P, Benali L (2007) Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 253:180–204
Schaff U, Sommer G (2011) Whole blood immunoassay based on centrifugal bead sedimentation. Clin Chem 57:753–761
Sharma R, Nandakumar K (1995) Flow through rotating rectangular ducts. Phys Fluids 7:1568–1575
Siegrist J, Gorkin R, Bastien M, Stewart G, Peytavi R, Kido H, Bergeron M, Madou M (2010) Validation of a centrifugal microfluidic sample lysis and homogenization platform for nucleic acid extraction with clinical samples. Lab Chip 10:363–371
Sollier E, Rostainga H, Pouteaua P, Fouilleta Y, Achard JL (2009) Passive microfluidics devices for plasma extraction from whole human blood. Sens Actuators B Chem 141:617–624
Stott SL, Toner M et al (2010) Isolation of circulating tumor cells using a microvortex generating herringbone chip. PNAS 107:18392–18397
Stroock A, Dertinger S, Ajdari A, Mezic I, Stone H, Whitesides G (2002) Chaotic mixer for microchannels. Science 295:647
Sudarsan AP, Ugaz V (2006) Multivortex micromixing. PNAS 103(19):7228–7233
Tibbe A, Miller M, Terstappen L (2007) Statistical considerations for enumeration of circulating tumor cells. Cytom A 71:154–162
Tsai H Jr, Lin L (2002) Active microfluidic mixer and gas bubbler driven by thermal bubble micropump. Sens Actuators A Phys 97:665–671
Vijayendran R, Motsegood K, Beebe D, Leckband D (2003) Evaluation of a three-dimensional micromixer in a surface-based biosensor. Langmuir 19:1824–1828
Voldman J, Gray J, Schmidt J (2000) An integrated liquid mixer/valve. Microelectromech Syst 9:295–302
Wang H, Iovenitti P, Harvey E, Masood S (2002) Optimizing layout of obstacles for enhanced mixing in microchannels. Smart Mater Struct 11:662
Wong S, Ward M, Wharton C (2004) Micro T-mixer as a rapid mixing micromixer. Sens Actuators B 100:365–385
Xin W, Chen X, Mab X, Kong X, Xua Z, Wang J (2011) Fast DNA hybridization on a microfluidic mixing device based on pneumatic driving. Talanta 84:565–571
Yang Z, Matsumoto S, Goto H, Matsumoto M, Maeda R (2001) Ultrasonic micromixer for microfluidic systems. Sens Actuators A Phys 93:266–272
Zhang J, Guo Q, Liu M, Yang J (2008) A lab-on-CD prototype for high-speed blood separation. J Micromech Microeng 18:125025
Zhang J, Li W, Li M, Alici G, Nguyen N-T (2013) Particle inertial focusing and its mechanism in a serpentine microchannel. Microfluid Nanofluid. doi:10.1007/s10404-013-1306-6
Acknowledgments
Authors would like to thank Dr Macdara Glynn for supplying MCF7 cells. This material is based upon works supported by the Science Foundation Ireland under Grant No. 10/CE/B1821.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (M4 V 2867 kb)
Supplementary material 2 (M4 V 2215 kb)
Supplementary material 3 (M4 V 5098 kb)
Rights and permissions
About this article
Cite this article
Aguirre, G.R., Efremov, V., Kitsara, M. et al. Integrated micromixer for incubation and separation of cancer cells on a centrifugal platform using inertial and dean forces. Microfluid Nanofluid 18, 513–526 (2015). https://doi.org/10.1007/s10404-014-1450-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1450-7