Abstract
Purpose
To determine the capabilities of the “Kago-Eye2” software to semi-automatically segment the choroid in the optical coherence tomographic (OCT) images.
Study design
A cross-sectional, prospective study of 44 healthy volunteers.
Methods
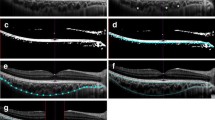
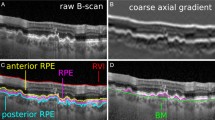
The Kago-eye2 software was developed to detect the border between Choriocapillaris and Sattler’s layer (C–S) and between Sattler’s layer and Haller’s layer (S–H). The intra- and inter-grader agreements were determined for the segmentations made with semi-automated and manual analysis using the Kago-Eye2 software. The inter-method agreements were determined for two independent graders.
Results
Forty-four right eyes of 44 heathy volunteers (22 men) with a mean age of 35.0 ± 8.8 years were studied. The intra-grader agreement of the C–S border was 0.97 for grader 1 and 0.892 for grader 2 for the manual segmentation, and 0.908 for grader 1 and 0.842 for grader 2 for the Kago-Eye2 segmentation. For the S–H border, the intra-grader agreement was 0.96 for grader 1 and 0.981 for grader 2 for manual segmentation and 0.855 for grader 1 and 0.839 for grader 2 with the Kago-Eye2. For the C–S and S–H border, the inter-grader agreement was 0.548 and 0.902 for manual segmentation and 0.947 and 0.833 for the Kago-Eye2. The inter-method agreement was 0.565 for the C–S border and 0.759 for the S–H border.
Conclusion
The Kago-Eye2 software can segment the layers of the subfoveal choroid with good reproducibility and repeatability. We conclude that the Kago-Eye2 software can be used for semi-automatic segmentation of the choroidal layers.



Similar content being viewed by others
References
Castro-Correia J. Understanding the choroid. Int Ophthalmol. 1995;19:135–47.
Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–73.
Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res. 2010;29:19–29.
Lutty GA, Cao J, McLeod DS. Relationship of polymorphonuclear leukocytes to capillary dropout in the human diabetic choroid. Am J Pathol. 1997;151:707–14.
Saito M, Kano M, Itagaki K, Ise S, Imaizumi K, Sekiryu T. Subfoveal choroidal thickness in polypoidal choroidal vasculopathy after switching to intravitreal aflibercept injection. Jpn J Ophthalmol. 2016;60:35–41.
Yoshikawa M, Akagi T, Nakanishi H, Ikeda HO, Morooka S, Yamada H, et al. Longitudinal change in choroidal thickness after trabeculectomy in primary open-angle glaucoma patients. Jpn J Ophthalmol. 2017;61:105–12.
Pablo LE, Bambo MP, Cameo B, Ferrández B, Güerri N, Polo V, et al. The use of zonal analysis of peripapillary choroidal thickness in primary open-angle glaucoma. Jpn J Ophthalmol. 2018;62(1):41–7.
Song YJ, Kim YK, Jeoung JW, Park KH. Assessment of peripapillary choroidal thickness in primary open-angle glaucoma patients with choroidal vascular prominence. Jpn J Ophthalmol. 2017;61:448–56.
Enaida H, Nagata S, Takeda A, Nakao S, Ikeda Y, Ishibashi T. Changes in chorioretinal blood flow velocity and cerebral blood flow after carotid endarterectomy. Jpn J Ophthalmol. 2016;60:459–65.
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Kim JH, Kang SW, Kim JR, Kim SJ. Variability of subfoveal choroidal thickness measurements in patients with age-related macular degeneration and central serous chorioretinopathy. Eye (Lond). 2013;27:809–15.
Rayess N, Rahimy E, Ying GS, Bagheri N, Ho AC, Regillo CD, et al. Baseline choroidal thickness as a predictor for response to anti-vascular endothelial growth factor therapy in diabetic macular edema. Am J Ophthalmol. 2015;159(85–91):e81–3.
Sonoda S, Sakamoto T, Otsuka H, Yoshinaga N, Yamashita T, Kii Y, et al. Responsiveness of eyes with polypoidal choroidal vasculopathy with choroidal hyperpermeability to intravitreal ranibizumab. BMC Ophthalmol. 2013;13:43.
Sonoda S, Sakamoto T, Yamashita T, Shirasawa M, Uchino E, Terasaki H, et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 2014;55:3893–9.
Sonoda S, Sakamoto T, Yamashita T, Uchino E, Kawano H, Yoshihara N, et al. Luminal and stromal areas of choroid determined by binarization method of optical coherence tomographic images. Am J Ophthalmol. 2015;159(1123–31):e1121.
Sonoda S, Sakamoto T, Kakiuchi N, Shiihara H, Sakoguchi T, Tomita M, et al. Semi-automated software to measure luminal and stromal areas of choroid in optical coherence tomographic images. Jpn J Ophthalmol. 2017 (in press).
Kinoshita T, Mitamura Y, Mori T, Akaiwa K, Semba K, Egawa M, et al. Changes in choroidal structures in eyes with chronic central serous chorioretinopathy after half-dose photodynamic therapy. PLoS One. 2016;11:e0163104.
Sonoda S, Sakamoto T, Kuroiwa N, Arimura N, Kawano H, Yoshihara N, et al. Structural changes of inner and outer choroid in central serous chorioretinopathy determined by optical coherence tomography. PLoS One. 2016;11:e0157190.
Shirasawa M, Sakamoto T, Terasaki H, Yamashita T, Uchino E, Sonoda S. Objective determination of optimal number of spectral-domain optical coherence tomographic images of retina to average. PLoS One. 2014;9:e110550.
Yamashita T, Yamashita T, Shirasawa M, Arimura N, Terasaki H, Sakamoto T. Repeatability and reproducibility of subfoveal choroidal thickness in normal eyes of Japanese using different SD-OCT devices. Invest Ophthalmol Vis Sci. 2012;53:1102–7.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68.
Adhi M, Ferrara D, Mullins RF, Baumal CR, Mohler KJ, Kraus MF, et al. Characterization of choroidal layers in normal aging eyes using enface swept-source optical coherence tomography. PLoS One. 2015;10:e0133080.
Almeida DR, Zhang L, Chin EK, Mullins RF, Kucukevcilioglu M, Critser DB, et al. Comparison of retinal and choriocapillaris thicknesses following sitting to supine transition in healthy individuals and patients with age-related macular degeneration. JAMA Ophthalmol. 2015;133:297–303.
Fleckenstein M, Charbel Issa P, Helb HM, Schmitz-Valckenberg S, Finger RP, Scholl HP, et al. High-resolution spectral domain-OCT imaging in geographic atrophy associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:4137–44.
Hassenstein A, Meyer CH. Clinical use and research applications of Heidelberg retinal angiography and spectral-domain optical coherence tomography - a review. Clin Exp Ophthalmol. 2009;37:130–43.
Acknowledgements
The authors thank Professor Emeritus Duco Hamasaki of the Bascom Palmer Eye Institute of the University of Miami for providing critical discussions and suggestions to our study and revision of the final manuscript. This study was supported by JSPS KAKENHI Grant number 15H04996.
Funding
The funding organizations had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors has any conflict of interest in any materials, software or methods in this manuscript.
Additional information
Corresponding author: Taiji Sakamoto
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sonoda, S., Terasaki, H., Kakiuchi, N. et al. Kago-Eye2 software for semi-automated segmentation of subfoveal choroid of optical coherence tomographic images. Jpn J Ophthalmol 63, 82–89 (2019). https://doi.org/10.1007/s10384-018-0631-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-018-0631-4