Abstract
Purpose
To compare the effectiveness and safety of either switching from topical prostaglandin (PG) analog monotherapy to topical PG/timolol fixed combination therapy or adding topical ripasudil therapy.
Study design
An open-label, prospective, randomized, parallel group, comparative study
Methods
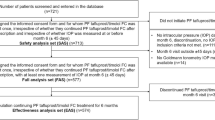
Fifty-one patients (51 eyes) with primary open-angle glaucoma who experienced insufficient intraocular pressure (IOP) control while taking a PG analog were enrolled. The participants were divided into the following treatment groups: PG/timolol fixed combination (switched group) or ripasudil therapy addition (added group). Blood pressure, IOP, and pulse rate were measured at baseline and after 1 and 3 months of study treatment. Adverse reactions and decreased effectiveness were examined.
Results
The mean IOP after 3 months of therapy was 14.3 ± 2.2 mmHg in the switched group and 14.7 ± 3.0 mmHg in the added group, both of which were significantly lower than those at baseline (switched, 16.3 ± 3.0 mmHg; added, 16.6 ± 2.8 mmHg; both P < .001). At 3 months, the IOP was reduced by 2.0 ± 1.7 mmHg (11.7 ± 9.6%) in the switched group and by 1.8 ± 2.1 mmHg (10.7 ± 12.5%) in the added group. In the added group, the diastolic blood pressure after 1 month of therapy was significantly lower than that at baseline (P < .05). In the switched group, 10 (40.0%) and 2 (8.0%) participants experienced adverse reactions at 1 and 3 months, respectively. In the added group, 6 (23.1%) and 4 (15.4%) participants experienced adverse reactions at 1 and 3 months, respectively. Treatment was discontinued in 4 participants (16.0%) in the switched group and in 1 participant (3.8%) in the added group.
Conclusion
Treatment changes involving either switching from a PG analog to PG/timolol fixed combination eye drops or adding ripasudil to PG analog therapy were equally safe and effective in reducing IOP.


Similar content being viewed by others
References
Schmier JK, Hulme-Lowe CK, Covert DW. Adjunctive therapy patterns in glaucoma patients using prostaglandin analogs. Clin Ophthalmol. 2014;8:1097–104.
Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, et al. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–44.
Isobe T, Mizuno K, Kaneko Y, Ohta M, Koide T, Tanabe S. Effects of K-115, a rho-kinase inhibitor, on aqueous humor dynamics in rabbits. Curr Eye Res. 2014;39:813–22.
Inoue K, Okayama R, Shiokawa M, Ishida K, Tomita G. Efficacy and safety of adding ripasudil to existing treatment regimens for reducing intraocular pressure. Int Ophthalmol. 2017. https://doi.org/10.1007/s10792-016-0427-9.
Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Suganami H, et al. Additive intraocular pressure-lowering effects of the Rho kinase inhibitor Ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 2015;133:755–61.
Tanihara H, Inoue T, Yamamoto T, Kuwayama Y, Abe H, Fukushima A, et al. One-year clinical evaluation of 0.4% ripasudil (K-115) in patients with open-angle glaucoma and ocular hypertension. Acta Ophthalmol. 2016;94:e26–34.
Kohmoto R, Sugiyama T, Kojima S, Ueki M, Ikeda T. Optic nerve head blood flow changes induced by ripasudil added to prostaglandin analogues in primary open angle glaucoma. EC Ophthalmol. 2017;4:640–7.
Matsumura R, Inoue T, Matsumura A, Tanihara H. Efficacy of ripasudil as a second-line medication in addition to a prostaglandin analog in patients with exfoliation glaucoma: a pilot study. Clin Drug Invest. 2017;37:535–9.
Miyata K, Amano S, Sawa M, Nishida T. A novel grading method for superficial punctate keratopathy magnitude and its correlation with corneal epithelial permeability. Arch Ophthalmol. 2003;121:1537–9.
Hamacher T, Schinzel M, Schölzel-Klatt A, Neff HM, Maier H, Schlaffer G, et al. Short term efficacy and safety in glaucoma patients changed to the latanoprost 0.005%/timolol maleate 0.5% fixed combination from monotherapies and adjunctive therapies. Br J Ophthalmol. 2004;88:1295–8.
Dunker S, Schmucker A, Maier H, Latanoprost/Timolol Fixed Combination Study Group. Tolerability, quality of life, and persistency of use in patients with glaucoma who are switched to the fixed combination of latanoprost and timolol. Adv Ther. 2007;24:376–86.
Polo V, Larrosa JM, Ferreras A, Borque E, Pablo LE, Honrubia FM. Effect on diurnal intraocular pressure of the fixed combination of latanoprost 0.005% and timolol 0.5% administered in the evening in glaucoma. Ann Ophthalmol. 2008;40:157–62.
Kitazawa K, KP2035 Study Group. Phase 3 double-blind study of latanoprost/timolol combination (KP2035) in patients with primary open-angle glaucoma or ocular hypertension [in Japanese]. Rinsho Ganka. 2009;63:807–15.
Inoue K, Okayama R, Higa R, Wakakura M, Tomita G. Assessment of ocular hypotensive effect and safety 12 months after changing from an unfixed combination to a latanoprost 0.005% + timolol maleate 0.5% fixed combination. Clin Ophthalmol. 2012;6:607–12.
Mandic Z, Novak-Lauš K, Bojic L, Popovic-Suic S, Maricic-Došen V, Pelcic G, et al. Observational study of patients switched to the fixed travoprost 0.004%/timolol 0.5% combination in Croatia. Methods Find Exp Clin Pharmacol. 2010;32:593–8.
Pfeiffer N, Scherzer ML, Maier H, Schoelzel S, Jasek MC, Stewart JA, et al. Safety and efficacy of changing to the travoprost/timolol maleate fixed combination (DuoTrav) from prior mono- or adjunctive therapy. Clin Ophthalmol. 2010;14:459–66.
Costa VP, Moreira H, Paolera MD, de Moraes Silva MR. Efficacy and safety of travoprost 0.004%/timolol 0.5% fixed combination as transition therapy in patients previously on prostaglandin analog monotherapy. Clin Ophthalmol. 2012;6:699–706.
Muraki T, Inoue K, Ishida K, Tomita G. Long-term outcome of switching from travoprost to combination of travoprost and timolol [in Japanese]. Rinsho Ganka. 2015;69:1493–8.
Kuwayama Y, DE-111 Study Group. Phase III double-masked study of fixed combination tafluprost 0.0015%/timolol 0.5% (DE-111) versus tafluprost 0.0015% alone or given concomitantly with timolol 0.5% in primary open angle glaucoma and ocular hypertension [in Japanese]. Atarashii Ganka. 2013;30:1185–94.
Kuwayama Y, DE-111 Study Group. A long-term, open-label study of fixed combination tafluprost 0.0015%/timolol 0.5% (DE-111) in patients with open-angle glaucoma or ocular hypertension [in Japanese]. Atarashii Ganka. 2015;32:113–43.
Pillunat LE, Erb C, Ropo A, Kimmich F, Pfeiffer N. Preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% in patients with open-angle glaucoma and ocular hypertension: results of an open-label observational study. Clin Ophthalmol. 2017;11:1051–64.
Terao E, Nakakura S, Fujisawa Y, Fujio Y, Matsuya K, Kobayashi Y, et al. Time course of conjunctival hyperemia induced by a Rho-kinase inhibitor anti-glaucoma eye drop: Ripasudil 0.4. Curr Eye Res. 2017;42:738–42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K. Inoue, None; K. Ishida, None; G. Tomita, None.
About this article
Cite this article
Inoue, K., Ishida, K. & Tomita, G. Effectiveness and safety of switching from prostaglandin analog monotherapy to prostaglandin/timolol fixed combination therapy or adding ripasudil. Jpn J Ophthalmol 62, 508–516 (2018). https://doi.org/10.1007/s10384-018-0599-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-018-0599-0