Abstract
Monitoring carnivore populations requires sensitive and trustworthy assessment methods to make reasonable and effective management decisions. The Eurasian fish otter Lutra lutra experienced a dramatic population decline throughout Europe during the twentieth century but is currently recovering in both distribution range and population size. In Austria, most assessments on otter distribution have applied a modified version of the so-called “British” or “standard” method utilizing point-wise surveys for otter spraints at predefined monitoring bridges. In this study, we synthesize several recent statewide assessments to compile the current otter distribution in Austria and evaluate the efficiency and sensitivity of the “monitoring bridge” approach in comparison to the “standard” method. The otter shows an almost comprehensive distribution throughout eastern and central Austria, while more western areas (Tyrol and Vorarlberg) are only partially inhabited, likely due to a still ongoing westward expansion. Furthermore, the bridge monitoring method utilizing presence/absence information on otter spraints reveals itself to be a time- and cost- effective monitoring tool with a tolerable loss of sensitivity for large-scale otter distribution assessments. Count data of spraints seem to be prone to observer bias or environmental influences like weather or flooding events making them less suitable for quantitative analyses.
Similar content being viewed by others
Introduction
Carnivores are known to be particularly sensitive to human disturbances due to their usually large home ranges, low population densities, long generation times, and low fecundity (Woodroffe 2000; Purvis et al. 2000; Baker and Leberg 2018) but also because of direct persecution (Purvis et al. 2000; Treves and Karanth 2003). Consequently, areas of high human population density and intense land use, such as central Europe, have had their carnivore populations reduced or depleted (Ripple et al. 2014; Chapron et al. 2014). However, due to protective legislation, changes in public perception, and successful implementation of conservation practices, European countries are currently experiencing a stabilization or even expansion of various carnivore species. While from a conservation perspective, these developments are fundamentally positive; they may also trigger increased human-wildlife conflicts in these areas (Breitenmoser 1998; Liordos et al. 2019). The conflicting objectives among stakeholders involving conservation, agriculture, fisheries, and wildlife management and the embedding of carnivore management in a broader political, socioeconomic, and emotional context make monitoring their populations a particularly sensitive and complicated challenge that requires efficient, objective, and trustworthy methodologies to inform management decisions (Ripple et al. 2014; Chapron et al. 2014; Liordos et al. 2019).
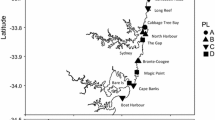
The Eurasian fish otter Lutra lutra (hereafter “otter”) experienced a dramatic population decline throughout Europe during the twentieth century (Macdonald and Mason 1994; Roos et al. 2015), attributed to direct persecution, deterioration, or loss of habitat and/or pollution such as with polychlorinated biphenyls (Mason and Macdonald 1986; Foster-Turley et al. 1990; Macdonald and Mason 1994; Roos et al. 2015). Becoming rare or extinct in most central European countries in the second half of the twentieth century, the otter was included in Annex II and Annex IV of the EU Habitats Directive (Council Directive 92/43/EEC). In Austria, the otter received initial legal protection in the 1940s and since 1994; it is listed as NT (near threatened) in Austria’s “Red list of endangered species” (Spitzenberger 2005). The otter was presumably continuously present throughout the twentieth century only in two refuge areas located along border regions of neighboring countries: (A) in northern Austria in the states Upper and Lower Austria bordering the Czech Republic, and (B) in the southeast of Austria in the states of Styria and Burgenland bordering Slovenia and Hungary (Fig. 1). Information on otter presence during the mid-twentieth century heavily relies on hunting reports, museum materials or regional assessments (e.g., Jahrl 1995, 2001) and the first nation-wide attempts to map otter distribution were conducted in the 1990s. These mapping efforts summarized data of various regional studies and showed a clear clustering of frequent otter records in the two presumed refuge areas (Gutleb 1994; Jahrl 1999; Fig. 1). Between the mid-1990s and 2020s, multiple state wide-assessments targeting current otter distribution or population size estimations were conducted throughout Austria, documenting a clear re-expansion during the 2010s and 2020s. Based on these assessments, the otter is currently distributed across the whole of Upper and Lower Austria, Styria, Carinthia, Salzburg and Burgenland, and a small area of Tyrol with only Vorarlberg reporting no occurrence (Kranz and Poledník 2013a, 2020; Kofler et al. 2018; Holzinger et al. 2020; Schenekar and Weiss 2020, 2021a, b). While the latest of these assessments incorporated genetic tools for otter population size estimations, distribution assessment is solely based on detection of otter spraints (otter scat or anal jelly) at predefined monitoring sites, mostly bridges across running waters.

Modified from Jahrl (1999)
Summary of otter reports in Austria between 1990 and 1998. Data combines systematic assays and opportunistic records from multiple resources during that time period. Full black circles represent a high number of records during that time, whereas half-full circles indicate only rare or occasional records. Empty circles indicate negative survey results only. The approximate location of two otter refuge areas (A and B) is indicated by dashed circles. Inset shows locations of the nine states of Austria. VO, Vorarlberg; TI, Tyrol; SA, Salzburg; CA, Carinthia; UA, Upper Austria; LA, Lower Austria; ST, Styria; VI, Vienna; BL, Burgenland.
Assessments of otter presence in Austria generally follow a modified version of the otter survey method, commonly termed “standard” or “British” method, originally developed to assess otter distribution on the British Isles in the 1970s and 1980s (Macdonald 1983). Because modified versions of this method have been adapted in multiple countries with concerns being raised about varying sensitivity or the comparability across studies (O’Sullivan 1993; Romanowski et al. 1996; Romanowski and Brzezinski 1997; Reuther et al. 2000; Chanin 2003; Reid et al. 2013, 2014), a standard protocol has been defined by Reuther et al. (2000). This standard method recommends four predefined survey sites (usually river stretches but also coast lines of lakes or the sea) on a 10 × 10-km UTM square grid. At each site, one bank is searched for signs of otters (spraints or footprints), for a total length of up to 600 m. If signs of otters are found before reaching the total length, the search is terminated. The overall result of each grid cell as well as the percentage of otter-positive sites within a cell is reported.
In Austria, such bridge/grid surveys follow Reuther et al. (2000), except that instead of surveying a river stretch up to 600 m along one bank, only the immediate area under a bridge is surveyed, but on both sides of the river. This deviation was initially justified by the advantage of the spraint being protected from rain or snow under the bridge and therefore being detectable for a longer period of time, a clearer definition of the survey sites and an overall higher time and cost efficiency per area unit (Kraus 1997; Kranz et al. 2003, 2005; Kranz and Poledník 2009a). This spot-check-like approach was previously suggested to be a “useful alternative” to the standard method (e.g., Chanin 2003). Furthermore, only otter spraints are counted as a record for otter presence but not tracks, presumably motivated by the comparability with previous studies but also due to a high risk of track mis-identification with other co-occurring mustelids (Kranz et al. 2003, 2005). Later surveys continued with the same methodology as requested by state governments contracting out such assessments (e.g., Kofler et al. 2018; Holzinger et al. 2020; Schenekar and Weiss 2020).
In this study, we aim to synthesize the latest statewide assessments of otter distribution for a current nation-wide distribution assessment of the otter in Austria and to evaluate and discuss the efficiency and suitability of the applied bridge monitoring approach for assessing otter distribution in comparison to the standard protocol suggested by Reuther et al. (2000).
Methods
Sources of field data
The field-derived data utilized in this study were originally produced in the framework of multiple statewide assessments of the otter population commissioned by the respective state governments. Thus, individual field data sets derived from Styria (Holzinger et al. 2020), Carinthia (Schenekar and Weiss 2020), Salzburg (Schenekar and Weiss 2021b), Upper Austria (Schenekar and Weiss 2021a), and Lower Austria (Kofler et al. 2018) were pooled for analysis. Additionally, distribution maps from assessments in Burgenland (Kranz and Poledník 2013a) and Tyrol (Kranz and Poledník 2020) were incorporated for the composition of a nation-wide distribution map.
Selection of survey sites (monitoring bridges)
Selection of monitoring bridges was carried out in the framework of prior studies of the above listed statewide assessments (Kranz and Poledník 2009a, b, c, 2012, 2013b). For initial selection, predefined bridges were classified on a multi-level scale (unsuitable, suitable, well suitable, and monitoring bridge) (e.g., Kraus 1997), which was later transferred into a “probability scale” of otters marking this bridge if present in the area (e.g., Kranz and Poledník 2012). A high marking probability was assumed when the area under the bridge (1) had structures used as preferred marking sites such as exposed rocks or stones, 2) was easily accessible for otters, (3) lacked signs of frequent human activity, and (4) exhibited a “cave-like” character based on vegetational growth (no vegetational cover equals a cave-like character) (Kranz and Poledník 2012, 2013c, 2020). For statewide surveys, only bridges with the highest possible probabilities were selected targeting on average four bridges per 10 × 10-km grid cell.
Collection of field data
All bridges were monitored during or close to winter months (November—March) and survey periods (time between first and last day of fieldwork) were aimed to be kept as short as possible. All surveys utilized professional field workers trained in otter spraint identification, except for the assessment in Upper Austria, which utilized volunteers of the provincial fisheries association who received training for otter spraint and track identification beforehand. For all surveys, the area under each monitoring bridge was checked for otter spraint on both sides of the river and the encountered spraint was classified into three categories of freshness: (A) fresh spraint: still wet, intact shape, characteristic smell, color not yet faded; (B) spraint of intermediate freshness: dry but not yet dissociated and color not yet lost; (C) old spraint: dry, dissociated and color faded. The total spraint counts as well as spraint counts in the three categories were noted. In Carinthia and Salzburg, spraint counts were capped at 50, and sites with > 50 were simply labeled has having “51 + ” spraints. Thus, counts from other regions were also capped at “51 + ” to standardize the data set. Additionally, measures on bridge dimensions were taken but collected data varied among the individual surveys (Table 1). Predefined, assessed bridges that were inaccessible during the survey period or were identified to be not suitable for spraint deposition were removed for analysis (34 bridges in Carinthia, 39 bridges in Upper Austria, 11 bridges in Salzburg). Furthermore, individual bridges that were surveyed far outside the survey period (Table 1) were also removed from analysis (four bridges in Styria, one in Salzburg, two bridges in Upper Austria). Finally, the reported GPS coordinates of 13 bridges (two in Carinthia, two in Lower Austria, one in Upper Austria and eight in Styria) could not be clearly assigned to a river of the Austrian total water network and thus were also removed. Overall, data from 2559 bridges were included in this analysis (Table 1).
Evaluation of the efficiency, sensitivity and suitability of the bridge monitoring method
To evaluate the efficiency and sensitivity of the point-wise bridge monitoring approach versus the stretch-wise standard method, we evaluated three aspects of the bridge monitoring method: (1) For method efficiency, we calculated the average number of surveyed sites per workday and per person for a subset of the Austrian data for which necessary information was available (data from Styria, Salzburg and Carinthia – 1263 monitoring bridges) and compared this to the average number of sites surveyed per workday and person reported from studies applying the standard method (data taken from Reuther et al. 2000); (2) for method sensitivity, for a subset of the Austrian data (Lower Austria – 794 bridges), up to 200 m along the water course was surveyed for otter spraints if the bridge itself did not reveal otter spraints. The percentage of otter-negative bridges at which additional spraints were found within the 20 m was calculated, serving as an indicator of efficacy/sensitivity, and (3) for suitability of the selected monitoring bridges, we compared bridge height, bridge width and river width between otter-positive and otter-negative bridges (via Wilcoxon rank sum test). Spatial analyses were all carried out in QGIS 3.10.0 – A Coruña (QGIS Development Team 2019). Field-recorded data of the monitoring bridges was converted to an ESRI-shape layer. Results of positive/negative monitoring bridges were overlayed with the 10 × 10-km UTM reference grid of the European Environmental Agency (EEA UTM reference grid, https://www.eea.europa.eu/data-and-maps/data/eea-reference-grids-2). Statistical analyses and plotting were all carried out using R 4.1.2 (R Core Team 2021) and the ggplot2 package (Wickham 2016).
Results
Otter distribution and spraint counts at monitoring bridges
Of the 2559 bridges surveyed, 2072 (81%) revealed at least one otter spraint and were therefore classified as otter “positive’ whereas at 487 bridges (19%), no otter spraint could be detected (otter “negative” bridges, Fig. 2a). Styria had the highest percentage of positive bridges (90.2%), followed by Lower Austria (83.6%), Upper Austria (75.5%), and Salzburg (61.9%). When converting the results onto the 10 × 10-km UTM reference grid, 641 grid cells had at least 50% of their area in the study area with an average of 3.8 monitoring bridges per grid cell. A total of 602 grid cells contained at least one monitoring bridge. Of these, 574 (95.4%) contained at least one otter positive site, whereas 28 (4.6%) contained only otter negative sites (Fig. 2b). Total counts ranged from 0 to 161 spraints per site (with a Poisson-like distribution, mean: 11.6 median: 6, Fig. S1a, Online Resource 1), with Styria having the highest average spraint count (mean: 22.6; median: 15) and Upper Austria having the lowest average spraint count (mean: 5.7; median: 3). Salzburg had the lowest median (mean: 5.9; median: 2). When counting only fresh spraints (category A), the maximum was 16 spraints per site (Fig. S1b, Online Resource 1). At 1772 of 2559 bridges (69.2%), no fresh spraints were found. At the 787 sites where fresh spraints were found, the overall median number of spraints was two (with Carinthia, Salzburg and Upper Austria having a median of two and Lower Austria and Styria having a median of one). The total spraint count and fresh spraint count were significantly correlated (Spearman correlation, R = 0.34, P < 0.01, Fig. S2, Online Resource 2).
Combined results of the monitoring surveys in five different states of Austria utilizing monitoring bridges as survey sites. a Results of the individual bridges. Black circles indicate bridges at which at least one otter spraint was found, white circles indicate bridges where no otter spraints were found. White circles are presented semi-transparent when overlapping with other datapoints for better visualization of the complete dataset. b Results converted onto 10 × 10-km EEA UTM reference grid. Black grid cells indicate cells that contain at least one positive monitoring bridge, gray grid cells indicate grid cells that contain only negative monitoring bridges. White grid cells do not contain any monitoring bridge. Data was available for the five Austrian states Carinthia, Salzburg, Styria, Lower Austria and Upper Austria, whereas no data was available for Vorarlberg, Tirol, Vienna, and Burgenland (shaded in gray in a)
Evaluation of the bridge monitoring method
Efficiency and time budget
In Carinthia, Styria, and Salzburg, surveying the 1263 bridges took a total of 90 workdays in the field, either in teams of two or surveyors working individually. When working in a team of two, 184 bridges could be surveyed within 10 workdays in the field (equaling to 9.2 sites per day per surveyor) and when working individually, 1079 sites could be surveyed within 80 (equaling to 13.5 sites per day per surveyor).
Reuther et al. (2000) reports the average number of sites surveyed by the standard method per workday and per surveyor of multiple studies. The number of sites per work day and surveyor was 5.6 sites in Portugal (with 1.02 survey sites per 10 × 10 km2), 7.2, 7.7, and 11.0 sites in three surveys in Sweden (with an average of 2.5 survey sites per 10 × 10 km2) and 25 sites in survey in Scotland (3.9 survey sites per 10 × 10 km2), whereby the latter included workdays ranging from 6:00 to 20:00 h. Overall, Reuther et al. (2000) estimated that approximately seven sites could be surveyed per day and per person when applying the standard method if the sites have to be searched for the full 600 m.
Sensitivity
From the Lower Austria dataset, out of the 794 bridges surveyed, 130 did not reveal otter spraints directly at the bridges. When searching up to 200 m along the riverbank for otter spraint, 13 of these survey sites (10%) revealed additional otter spraints.
Two hundered meters still represents a shorter distance searched than the recommended length by the standard method (600 m) and could therefore underestimate the percentage of positive bridges that could additionally be identified. Brzeziński (1991) found 87% of otter signs within the first 200 m searched in the Bieszczady mountains in Poland and a national survey in Poland found 90.3% of the otter signs within the first 200 m searched (Reuther et al. 2000), suggesting only a mild underestimation (efficiency 10%) when searching 200 m instead of 600 m. Therefore, the observed “increased false negative rate” due to only surveying bridges in a point-wise manner compared to surveying 600-m-long stretches can be combined to 11% per survey site (10% of newly positive bridges when searching 200 m × 1.10 due to 10% underestimation when searching 200 m compared to searching 600 m). Therefore, the combined risk of receiving a false negative 10 × 10 km2 when aiming to survey four bridges per square is 0.015% (11%4), leading to a negligible loss of accuracy on the 10 × 10-km grid level. Kofler et al. (2018) reported one 10 × 10 km2 out of 211 10 × 10 km2 surveyed (0.5%) that had a change in otter status when including data from the 200-m searches compared to the data only collected directly at bridges.
Suitability of selected monitoring bridges
All three parameters concerning bridge morphology differed significantly between otter-positive (at least one spraint found at a bridge) and otter-negative bridges (no spraint found) (Wilcoxon rank sum tests, W = 48,798, 177,448, and 351,474 for bridge height, bridge width, and river width, respectively, all P < 0.001). Thereby, otter-positive bridges had overall lower but broader bridges at narrower rivers (Fig. 3, Table 2). However, otter-positive bridges were found throughout the whole range of the three parameters, including the highest bridge surveyed (13 m) and the widest river (187 m) and the second widest bridge (180 m). Furthermore, no clear trend in increase of negative bridges could be observed with increasing bridge height, decreasing bridge width or increasing river width (Fig. S3, Online Resource 2).
Boxplot/violin plots comparing measures of the monitoring bridges at which no otter spraints were found (negative) and bridges at which at least one otter spraint was found (positive). *** indicates a significant difference (Wilcoxon rank sum test, P < 0.001). For bridge width and river width, three and two outlier data points, respectively, were removed for better visualization
Discussion
Current distribution of the otter in Austria
The combined results of the individual surveys of the five states in Austria suggest a nearly exhaustive distribution (95.4%) of the otter across the study area at the 10 × 10-km scale. Negative grid cells were mostly individually scattered across the study area with less than four data points inside them. The only exception is the accumulation of negative cells around the western border range between Salzburg and Carinthia, a mountainous area encompassing the High Tauern mountain range, including the highest mountain peaks (up to 3800 m) in Austria. This area likely represents limited otter habitat and contains potentially inaccessible valleys (such as due to steep waterfalls) for the otter. The two adjacent negative grid cells in Lower Austria fall into heavily industrialized areas around the cities Linz and Wels, also representing limited otter habitat. Across the study area, we failed to detect a significant influence of most potential environmental predictors and otter spraint presence or counts (Online Resource 3). The combined results of this study together with the limited data from Burgenland and Tyrol (Kranz and Poledník 2013a, 2020) show relatively exhaustive colonization of the otter in eastern and central Austria, with only partial colonization in western Austria, likely due to ongoing westward colonization. No recent data is available for the states of Vienna and Vorarlberg (Fig. 4).
Current distribution of the Eurasian fish otter in Austria combining results from this study and results from Kranz and Poledník (2020) for Tyrol and from Kranz and Poledník (2013a) for Burgenland. Please note that this study utilized the EEA UTM reference grid while previous studies utilized the UTM grid of Reuther et al. (2000)
Evaluation of bridge monitoring method compared to the standard method
Variations of the “standard” method have been applied throughout Europe with point-wise methods (also termed “point checks”) also being applied in the very early days of systematic otter surveys (MacDonald and Mason 1976; Clayton and Jackson 1981; Jessop and Macguire 1990). Variations of the standard method can influence the efficiency and sensitivity of the survey and stakeholders need to be aware of these differences when planning studies or interpreting results (Romanowski and Brzezinski 1997; Reuther et al. 2000).
The number of sites (13.5) that can be surveyed per day and surveyor using this bridge monitoring method is almost double as high as the estimated seven sites per day estimated applying the standard method (Reuther et al. 2000) and having to search the full 600 m. The efficiency of the standard method is expected to increase with otter density (because the 600-m search along a bank can be terminated early when the first otter scat has been found) but the bridge monitoring method is more robust to such changes as accessing the bridge takes up most of the time spent during the survey, easing the calculation of time and costs spent on the survey. Notably, the achievable number of surveyed sites per day depends heavily on other factors affecting the time spent in the field, such as the distance between the survey sites, habitat structure, additional data being collected during the survey, weather conditions, and starting/living location of the surveyor (Reuther et al. 2000), making quantification of efficiency difficult. However, less time spent at individual sites allows surveying more sites per 10 × 10 km2, further increasing time efficiency of fieldwork and the accuracy of the overall distribution estimation. An additional advantage of surveying only relatively easily accessible point survey sites (almost every bridge is connected to a road) is the possibility of involving volunteers such as in citizen science projects, which is more difficult when fieldwork involves walking longer distances through difficult terrain containing, for example, steep river banks. While utilization of volunteers bears the risk of higher data quality fluctuations (Darwall and Dulvy 1996; Foster-Smith and Evans 2003; Loperfido et al. 2010), it can lead to major cost reductions. In Salzburg, Styria, and Carinthia (1138 surveyed bridges in total), approximately € 21.7 per bridge was spent using professional field workers adding considerable costs when hundreds of sites need to be surveyed for a monitoring project. In Upper Austria (490 bridges) and for an earlier study in Carinthia (345 bridges, Schenekar and Weiss 2018), bridge monitoring was carried out using unpaid volunteers delivering comprehensible results, as a follow-up study confirmed (Schenekar and Weiss 2020). Conducted 2.5 years later and employing professional field workers, the follow-up study showed an almost identical large-scale distribution pattern of the otter in Carinthia. In our experience, the success and data quality received when involving volunteers heavily depends on two main factors, namely good training and motivation/mobilization of the volunteers. The training of volunteers thereby needs to assure that the volunteers can accurately identify otter spraints. Given the distinct diet of the otter and corresponding distinct morphology of the otter spraints (oily and blackish appearance, often containing fish scales, bones, crayfish carapaces, or insect exoskeletons) combined with a very distinct musky or fishy smell (Kruuk 2006; Mason and Macdonald 2009), we estimate that reliable identification of otter spraints can be achieved after approximately an 1–2-h training unit with practical examples. Reliable aging of the spraints is more difficult as the altering of the spraints varies with weather condition and digested food. Therefore, we recommend not to work with volunteers when otter spraint aging is required. Motivation and mobilization can be achieved for example by involving volunteers that have an intrinsic interest or motivation in the outcomes of the survey and additionally through events like workshops or public presentations of results.
The significant difference of spraint presence at bridges that were lower, smaller, or at narrower rivers might support the hypothesis of otters preferring to mark bridges exhibiting a “cave-like” character, as well as the better protection of weather conditions at such bridges. However, in order to cover the complete river network of a study area equally, larger bridges on larger rivers also need to be surveyed. As otter spraint was found almost throughout the complete range of bridge height, width, and river width covered in this dataset, we conclude that the chosen bridges were suitable monitoring sites to reliably detect otters if present across the whole river network and area surveyed.
Finally, we note that Austria is very suitable for the bridge monitoring method because of its high density of roads as well as suitable bridges (Reuther et al. 2000). Such conditions may be less available in other countries, as it was the case with the British national otter survey.
Presence/absence vs. spraint count vs. fresh spraint count
The noticeable difference in total otter spraint count among states with Styria showing a remarkably high total spraint counts suggests the presence of strong observer bias among surveys including quantitative assessment of spraints, a problem clearly outlined in Reid et al. (2013). Furthermore, environmental conditions can influence total otter spraint counts if surveys have not been carried out in parallel or at the same time of the year. Flood events that raise water levels to a point that spraints get washed away have significant influence on total spraint count (Reid et al. 2013). This was potentially a problem for parts of the Upper Austria dataset in this study (with a flood event happening 14–21 days prior the survey) (Schenekar and Weiss 2021a) (Fig. S1). Therefore, survey managers should always try to the extent possible to avoid surveying during or soon after flooding events. Fresh spraint counts might be less influenced by environmental disturbances such as flood events but are even more prone to observer bias due to the difficulty of aging otter spraint reliably, as spraint morphology is affected by composition of the spraint and weather conditions such as temperature (e.g., frozen vs. thawed spraint) and humidity. Reliable and consistent aging of spraints seems difficult to achieve and could only be ensured by multiple day visits to the same site, as done in Sittenthaler et al. (2020), which might not be feasible for large-scale surveys. Taken together, we urge to only very carefully integrate otter spraint counts (both old and fresh spraints) in the estimation of otter abundances (if at all).
Conclusions
The Eurasian fish otter exhibits a nearly exhaustive distribution across the eastern and central regions of Austria (states Burgenland, Lower Austria, Upper Austria, Styria, Carinthia, and Salzburg, with no information available for Vienna) which generally fall in lower elevations within Austria. The only notable distribution gaps are around the High Tauern mountain range. Western Austria is only partially inhabited (parts of Tyrol) with no occurrence data reported from Vorarlberg. Overall, we conclude that the bridge monitoring method combined with utilizing only presence/absence data is a time- and cost-efficient alternative to the suggested standard method when assessing large-scale otter distribution. This approach has a great applicability and practicability when a large study area of about 10,000 km2 or more is targeted with a very tolerable potential decrease in overall sensitivity of distribution estimation.
Data availability
Raw data are available from the authors upon reasonable request and with permission of the respective state government of Austria.
References
Baker AD, Leberg PL (2018) Impacts of human recreation on carnivores in protected areas. PLoS ONE 13:e0195436. https://doi.org/10.1371/journal.pone.0195436
Breitenmoser U (1998) Large predators in the Alps: the fall and rise of man’s competitors. Biol Conserv 83:279–289. https://doi.org/10.1016/S0006-3207(97)00084-0
Brzeziński M (1991) Distribution of the river otter Lutra lutra L. in the Biesczady Mountains. Prz Zool 35:397–706
Chanin P (2003) Monitoring the otter Lutra lutra. Conserving Natura 2000 Rivers Monitoring Series No. 10
Chapron G, Kaczensky P, Linnell J et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519. https://doi.org/10.1126/science.1257553
Clayton C, Jackson M (1981) Norfolk Otter Survey 1980–981. Otters, J Otter Trust 1:16–22
Darwall WRT, Dulvy NK (1996) An evaluation of the suitability of non-specialist volunteer researchers for coral reef fish surveys. Mafia Island, Tanzania — a case study. Biol Conserv 78:223–231. https://doi.org/10.1016/0006-3207(95)00147-6
Foster-Smith J, Evans SM (2003) The value of marine ecological data collected by volunteers. Biol Conserv 113:199–213. https://doi.org/10.1016/S0006-3207(02)00373-7
Foster-Turley P, Macdonald S, Mason C (1990) Otters, an action plan for conservation
Gutleb AC (1994) Todesursachenforschung Fischotter: Grundlagen für ein Schutzkonzept von Lutra lutra L. 1758 - Bericht für die Jahre 1990–1992. Forschungsbericht Fischotter 2. Forschungsinstitut WWF Österreich 11:12–25
Holzinger W, Schenekar T, Weiss S, Zimmermann P (2020) Verbreitung und Bestand des Fischotters (Lutra lutra) in der Steiermark (Mammalia). Joannea Zoo 18:5–23
Jahrl J (1995) Historische und aktuelle Situation des Fischotters (Lutra lutra) und seines Lebensraumes in der Nationalparkregion Hohe Tauern. Mitt Haus Der Natur 12:29–77
Jahrl J (1999) Verbreitung des Eurasischen Fischotters (Lutra lutra) in Österreich, 1990–1998 (Mammalia). Joannea Zoo 1:5–12
Jahrl J (2001) Der Fischotter in Oberösterreich. ÖKO L 23:3–9
Jessop R, Macguire F (1990) Norfolk Otter Survey 1988/89: implications of the status of the otter. Otters, J Otter Trust 2:9–12
Kofler H, Lampa S, Ludwig T (2018) Fischotterverbreitung und Populationsgrößen in Niederösterreich 2018. Endbericht. ZT KOFLER Umweltmanagement im Auftrag des Amtes der Niederösterreichischen Landesregierung, 117 S
Kranz A, Poledník L (2009a) Fischotter - Verbreitung und Erhaltungszustand 2009 in Kärnten Endbericht im Auftrag der Abteilung 20 des Amtes der Kärntner Landesregierung
Kranz A, Poledník L (2009b) Fischotter – Verbreitung und Erhaltungszustand 2009 im Bundesland Salzburg. Endbericht im Auftrag der Abteilung 4 der Salzburger Landesregierung
Kranz A, Poledník L (2009c) Fischotter - Verbreitung und Erhaltungszustand 2008 in Niederösterreich. Endbericht im Auftrag der Abteilung Naturschutz des Amtes der Niederösterreichischen Landesregierung
Kranz A, Poledník L (2012) Fischotter - Verbreitung und Erhaltungszustand 2011 im Bundesland Steiermark. Endbericht im Auftrag der Fachabteilungen 10A und 13C des Amtes der Steiermärkischen Landesregierung
Kranz A, Poledník L (2013a) Fischotter im Burgenland: Verbreitung und Bestand 2013. Endbericht i.A.d. Naturschutzbundes Burgenland
Kranz A, Poledník L (2013b) Fischotter - Verbreitung und Erhaltungszustand 2012 in Oberösterreich. Endbericht im Auftrag der Abteilungen Natuschutz und Land- und Forstwirtschaft der Oberösterreichischen Landesregierung
Kranz A, Poledník L (2013c) Zum Fischotter: Lebensraum & Vorkommen in Osthessen. Analysen und ein Lokalaugenschein 2013 in Spessart un Rhön. Bericht im Auftrag des Regierungspräsidiums Darmstadt
Kranz A, Poledník L (2020) Fischotter in Tirol: Verbreitung und Bestand 2020. Endbericht im Auftrag des Amtes der Tiroler Landesregierung
Kranz A, Poledník L, Poledniková K (2003) Fischotter im Mühlviertel: Ökologie und Management Optionen im Zusammenhang mit Reduktionsanträgen. Gutachten im Auftrag des Oberösterreichischen Landesjagdverbandes, Hohenbrunn 1, A-4490 St. Florian. 73 Seiten
Kranz A, Poledník L, Toman A (2005) Aktuelle Verbreitung des Fischotters (Lutra lutra) in Kärnten und Osttirol. Carinthia II 195/115. J:317–344
Kraus E (1997) Fischotter-Kartierung Vorarlberg 1995. Vor Naturschau 3:9–46
Kruuk H (2006) Otters ecology, behaviour and conservation. Oxford University Press, Oxford
Liordos V, Kontsiotis VJ, Nevolianis C, Nikolopoulou CE (2019) Stakeholder preferences and consensus associated with managing an endangered aquatic predator: the Eurasian otter (Lutra lutra). Hum Dimens Wildl 24:446–462. https://doi.org/10.1080/10871209.2019.1622821
Loperfido JV, Beyer P, Just CL, Schnoor JL (2010) Uses and biases of volunteer water quality data. Environ Sci Technol 44:7193–7199. https://doi.org/10.1021/es100164c
Macdonald S, Mason C (1994) Status and conservation need of the otter (Lutra lutra) in the western Palaearctic. Council of Europe, Strassburg
MacDonald S, Mason C (1976) The status of the otter (Lutra lutra L.) in Norfolk. Biol Conserv 22:207–215
Macdonald SM (1983) The status of the otter (Lutra lutra) in the British Isles. Mamm Rev 13:11–23. https://doi.org/10.1111/j.1365-2907.1983.tb00260.x
Mason C, Macdonald S (1986) Otters: ecology and conservation. Cambridge University Press, Cambridge
Mason C, Macdonald S (2009) Otters: ecology and conservation. Cambridge University Press
O’Sullivan WM (1993) Efficiency and limitations of the standard otter (Lutra lutra) survey technique in Ireland. Biol Environ Proc R Irish Acad 93B:49–53
Purvis A, Gittleman J, Cowlishaw G, Mace G (2000) Predicting extinction risk in declining species. Proc Biol Sci 267:1947–1952. https://doi.org/10.1098/rspb.2000.1234
QGIS Development Team (2019) QGIS Geographic Information System. QGIS Association. http://www.qgis.org
R Core Team (2021) R: a language and environment for statistical computing
Reid N, Lundy MG, Hayden B et al (2013) Detecting detectability: identifying and correcting bias in binary wildlife surveys demonstrates their potential impact on conservation assessments. Eur J Wildl Res 59:869–879. https://doi.org/10.1007/s10344-013-0741-8
Reid N, Lundy MG, Hayden B et al (2014) Covering over the cracks in conservation assessments at EU interfaces: a cross-jurisdictional ecoregion scale approach using the Eurasian otter (Lutra lutra). Ecol Indic 45:93–102. https://doi.org/10.1016/j.ecolind.2014.03.023
Reuther C, Dolch D, Green S et al (2000) Surveying and monitoring distribution and population trends of the Eurasian otter (Lutra Lutra): guidelines and evaluation of the standard method for surveys as recommended by the European Section of the IUCN/SSC Otter Specialist Group. Gruppe Naturschutz
Ripple W, Estes J, Beschta R et al (2014) Status and ecological effects of the world’s largest carnivores. Science 343:1241484. https://doi.org/10.1126/science.1241484
Romanowski J, Brzezinski M (1997) How standard is the standard technique of the otter sruvey? IUCN Otter Spec Group Bull 14:57–61
Romanowski J, Brzezinski M, Cygan J (1996) Notes on the technique of the otter field survey. Acta Theriol (Warsz) 41:199–204. https://doi.org/10.4098/AT.arch.96-19
Roos A, Loy A, de Silva P et al (2015) Lutra lutra. The IUCN Red List of Threatened Species 2015.https://doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12419A21935287.en
Schenekar T, Weiss S (2018) Genetische Untersuchungen der Populationsgröße des Eurasischen Fischotters in den Kärntner Fischgewässern. Endbericht im Auftrag des Amts der Kärntner Landesregierung, 53 Seiten
Schenekar T, Weiss S (2021a) Studie zur Populationsgröße des Fischotters an den Fließgewässern Oberösterreichs. Endbericht im Auftrag des Amts der OÖ Landesregierung. 66 Seiten mit 2 Anhängen
Schenekar T, Weiss S (2021b) Studie zur Populationsgröße des Fischotters an den Salzburger Fließgewässern. Endbericht im Auftrag des Amts der Salzburger Landesregierung. 60 Seiten mit 2 Anhängen
Schenekar T, Weiss SJ (2020) Fischottermonitoring Kärnten 2019/2020. Endbericht im Auftrag des Amts der Kärntner Landesregierung. 43 Seiten mit einem Anhang
Sittenthaler M, Schöll EM, Leeb C et al (2020) Marking behaviour and census of Eurasian otters (Lutra lutra) in riverine habitats: what can scat abundances and non-invasive genetic sampling tell us about otter numbers? Mammal Res 65:191–202. https://doi.org/10.1007/s13364-020-00486-y
Spitzenberger F (2005) Rote Liste der Säugetiere Österreichs (Mammalia). In: Zulka KP, (Red.): Rote Listen gefährdeter Tiere Österreichs. Checklisten, Gefährdungsanalysen, Handlungsbedarf. Teil 1: Säugetiere, Vögel, Heuschrecken, Wasserkäfer, Netzflügler, Schnabelfliegen, Tagfalter. Grüne Reihe des Bundesministeriums für Land- . Gesamtherausgeberin Ruth Wallner, Böhlau, Wien, pp 45–62
Treves A, Karanth K (2003) Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv Biol 17:1491–1499. https://doi.org/10.1111/j.1523-1739.2003.00059.x
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Woodroffe R (2000) Predators and people: Using human densities to interpret declines of large carnivores. Anim Conserv 3:165–173. https://doi.org/10.1111/j.1469-1795.2000.tb00241.x
Acknowledgements
We would like to thank the involved departments of the provincial governments of Carinthia, Lower Austria, Salzburg, Styria, and Upper Austria as well as Hugo Kofler for provision of raw data, the fishing associations of Carinthia, Salzburg, and Upper Austria for their support, and the field workers for their commitments during data collection. The authors acknowledge the financial support by the University of Graz.
Funding
Open access funding provided by University of Graz.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by TS, SJW, and WEH. Data analysis was carried out by TS and AC. The first draft of the manuscript was written by TS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schenekar, T., Clark, A., Holzinger, W.E. et al. Presence of spraint at bridges as an effective monitoring tool to assess current Eurasian fish otter distribution in Austria. Eur J Wildl Res 68, 53 (2022). https://doi.org/10.1007/s10344-022-01604-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01604-8