Abstract
The redlegged earth mite, Halotydeus destructor, is an economically important pest of winter field crops and pastures in Australia, and has evolved field resistance to pyrethroid chemicals through a polymorphism in the voltage-gated parasodium channel leading to knockdown resistance (kdr). In this study, we quantified the rate of reversion to susceptibility of partially resistant H. destructor populations under field conditions in the absence of pesticide exposure. This was conducted over multiple years at two geographically distant locations with mite populations known to possess pyrethroid resistance. Fitness costs associated with pyrethroid resistance were identified through reductions in the frequency of kdr resistance alleles in the absence of pesticides. This was assessed using an amplicon sequencing approach targeting known resistance alleles. We also found that resistance can increase rapidly in frequency after only a single pyrethroid application in the field. Our results highlight that, once established in H. destructor populations, pyrethroid resistance will not easily be lost even after several years, emphasizing the importance of limiting the evolution of resistance in the first place. This helps to explain why pyrethroid resistance in H. destructor continues to persist at very high frequencies in the field and continues to expand within Australia despite the existence of fitness costs. Understanding field fitness costs associated with pesticide resistance is important when devising resistance management strategies for this pest.
Similar content being viewed by others
Key message
-
Fitness costs associated with the evolution of pesticide resistance in the redlegged earth mite were evaluated under field conditions.
-
Fitness costs were identified in the absence of pesticide exposure over a number of mite generations.
-
The findings from this study have implications for resistant management programs and, in particular, indicate chemical rotational strategies are important in mitigating future resistance issues in this pest.
Introduction
Mites (and other arthropods) utilise several biochemical, physiological and behavioural mechanisms to respond to toxic pesticides, which can lead to resistance evolving by genetic changes such as those involving constitutive overexpression of detoxification enzymes or mutations in target sites (Kliot and Ghanim 2012). These are often costly to an organism and can impact an individual’s fitness by decreasing its reproduction, survival, dispersal capability and/or its sensitivity to environmental conditions (Foster et al. 2003; Kliot and Ghanim 2012; Brito et al. 2013). Understanding fitness costs associated with pesticide resistance is important when devising resistance management strategies. Fitness costs can impact the evolution of resistance and the likelihood of resistant populations reverting to susceptibility in the absence of pesticides (Denholm and Rowland 1992). However, in practise there are relatively few cases of fitness costs being established in mite species that possess resistance.
In this paper, we assess field costs of resistance in the redlegged earth mite, Halotydeus destructor (Tucker) (Acari: Penthaleidae), one of the most economically important pests of winter field crops and pastures within Australia. The mite is highly polyphagous, attacking numerous plants including canola, cereals, medics, vegetables, flowers, pasture legumes, grasses and broadleaf weeds (Ridsdill-Smith 1997; Ridsdill-Smith et al. 2008). Halotydeus destructor is most damaging at the establishment phase of plant development in autumn; when present in large densities, feeding can result in plant death and substantial yield loss (Umina and Hoffmann 2004; Ridsdill-Smith et al. 2008). The mites reproduce sexually (Weeks et al. 1995), typically completing three generations a year (Ridsdill-Smith 1997; Cheng et al. 2018). Halotydeus destructor is found throughout areas of southern Australia with a Mediterranean-type climate (Ridsdill-Smith and Annells 1997; Robinson and Hoffmann 2001) where they are active from April to November. They undergo a summer diapause to survive hot dry summer conditions as an egg in the cadaver of adult females on the soil surface. Diapause eggs hatch the following autumn in response to sufficient rainfall and temperatures below 21 °C (Ridsdill-Smith and Annells 1997; McDonald et al. 2015).
Control of H. destructor is largely reliant on pesticides, through seed treatments or foliar sprays (Ridsdill-Smith et al. 2008). Of the five chemical Mode of Action (MoA) groups registered to control H. destructor in Australia, three (pyrethroids, organophosphates and neonicotinoids) are heavily used (Umina et al. 2019) and often applied prophylactically, thus creating high selection pressure for mites to evolve resistance. This, likely combined with the huge population sizes typical in the field (Ridsdill-Smith et al. 2008), has led to pesticide resistance issues in this pest (Umina 2007; Umina et al. 2012, 2017; Maino et al. 2018; Arthur et al. 2021). Populations of H. destructor resistant to organophosphates and/or pyrethroids are now present across large areas of Western Australia and parts of eastern Australia (Umina 2007; Umina et al. 2012, 2017; Maino et al. 2018; Arthur et al. 2021).
Pyrethroid resistance in H. destructor has been linked to a single-nucleotide polymorphism in the voltage-gated parasodium channel (Edwards et al. 2018) and is incompletely recessive (Cheng et al. 2019). The sodium channel has four homologous domains (I–IV), each containing six segments (S1–S6) (Rinkevich et al. 2013a), and genetic mutations in this channel which contribute to cross-resistance to DDT and pyrethroids are known as knockdown resistance (kdr) (Soderlund 2008). Pyrethroid resistance in H. destructor is associated with a novel amino acid change (L1024F), where leucine (L) is substituted with phenylalanine (F) in the II6 regions (Edwards et al. 2018). The genetic mechanism of organophosphate resistance remains unknown, although mutations within the acetylcholinesterase gene appear to be partly involved (O. Edwards, unpublished data).
Fitness costs associated with kdr-mediated pyrethroid resistance in H. destructor have previously been investigated under shade-house conditions (Cheng et al. 2021). Adult mites from resistant and susceptible field populations were mixed in varying proportions into microcosms; lower population fitness measures were demonstrated in treatments containing higher proportions of resistant mites in two out of three experiments, and the frequency of the kdr allele tended to decrease, suggesting a deleterious effect of the kdr resistance mutation on H. destructor fitness (Cheng et al. 2021). However, mite numbers maintained across generations within the microcosms were often low due to difficulties in culturing mites away from the field (Ridsdill-Smith 1991; Annells and Ridsdill-Smith 1994), and it was also not possible to interbreed populations for several generations before assessing mite fitness (see Ffrench-Constant and Bass 2017). Ideally fitness costs should be tested under field conditions given that temperature fluctuations and changing photoperiods, as well as other environmental conditions, are difficult to recreate in a shade-house but may influence fitness costs through pleiotropic effects of the kdr mutation.
Here, we quantified the rate of reversion to susceptibility of partially resistant H. destructor populations under field conditions without pesticide exposure. We addressed this by directly assessing fitness measures and changes in kdr allele frequencies of resistant mite populations using field plots. This was conducted over multiple years at two locations with known pyrethroid resistant mites. At one location, we also examined the impact of pyrethroid applications on H. destructor numbers and kdr allele frequencies.
2. Materials and methods
Field plot establishment
Two experimental field sites were established in different parts of Australia. One site was located in a pasture field (containing rye grass, Lolium perenne, medic, Medicago lupulina, subterranean clover, Trifolium subterraneum, serradella, Ornithopus sativus, and capeweed, Arctotheca calendula) near Arthur River (33.269 S, 116.950 E), in Western Australia. The second site was located in a perennial lucerne (Medicago sativa) field near Tintinara (35.943 S, 140.135 E), in South Australia. Each site was known to contain H. destructor populations with resistance to pyrethroid chemicals, with the Tintinara site also containing mites exhibiting organophosphate resistance (Maino et al. 2018).
In May 2017, a number of exclusion plots were constructed at each site following Weeks and Hoffmann (2000). These plots were constructed with white corflute sheeting (2.4 m × 1.22 m, 5 mm width) to form a 2 m × 2 m wide and 0.5–0.7 m high barrier. The sheeting was buried 30 cm into the ground using a mechanical trench digger to enhance durability (e.g., from wind) and minimise gaps along the lower edge of the barrier. Corner edges were fixed to wooden stakes with adhesive and reinforced with a staple gun. Tanglefoot® Insect Trap Coating Paste (The Tanglefoot Company, Michigan, USA) was applied to a 10 cm strip along the top outside edge of the barrier to prevent mites from entering or exiting each plot. At each field site, plots were set up approximately 1.5 m apart and separated from livestock. Exclusion plots have been successfully used previously in field experiments involving H. destructor to examine plant host associations, diapause strategies and competition (Umina and Hoffmann 2003, 2004, 2005).
Tracking mite numbers and resistance reversion
Twelve plots were constructed at each site to assess fitness costs of pyrethroid resistance in H. destructor across multiple years in the absence of pesticides. The initial kdr resistance allele frequency of mite populations within each plot was dictated by the frequency of field resistance at each location. This was determined by collecting mites and undertaking molecular screening (see below). A starting frequency of 50–80% was targeted in order to avoid fixation of susceptible or resistance alleles over the duration of the experiments. At Tintinara, the initial resistance levels were estimated at near 100%. To achieve a frequency of approximately 50% within each plot, we introduced susceptible mites which were collected in early June 2017 from a lucerne field approximately 150 m away from the field plots.
At each site, mites within each plot were sampled at intervals throughout the activity period of H. destructor between 2017 and 2020 (Table 1). At each sampling date, plants were assessed for feeding damage and mite numbers were recorded in each plot. After assessments at each sampling date, the vegetation within each plot was trimmed to a height of 20 cm with an Ozito® hedge trimmer (Ozito Industries, Victoria, Australia), and Tanglefoot® Insect Trap Coating Paste was reapplied to the outer edge where required. Sampling commenced later at the Arthur River site due to the later hatching of H. destructor from over-summering diapause eggs. Mite numbers were assessed via suction of four random samples in each plot within a 900 cm2 frame for 10 s using a Stihl SH55® blower vacuum (Stihl, Waiblingen, Germany) with a fine gauze mesh placed over the end of the vacuum tube. At the Tintinara site, each sample was emptied into a plastic tray and mites visually counted directly in the field. At Arthur River, mites were placed into 400 mL plastic screw top containers with the bottom replaced with fine wire mesh to allow air exchange. The containers were filled with plant material to maximise mite survival before being placed on ice for transport to the laboratory. These were stored overnight at 4 °C. The following day, the contents of each container were emptied into a plastic tray, and the number of mites counted visually.
At each sampling date, approximately 100 mites per plot (i.e., 25 mites from each of the four samples undertaken within each plot) were stored in Eppendorf tubes containing 100% ethanol and placed at − 20 °C for later molecular screening. Mites were screened fore known kdr mutations to determine kdr resistance allele frequencies (see below). If mite numbers within a plot were low (i.e., < 500 per m2) fewer than 100 mites were collected, or in some cases, not collected at all (see Fig. 1; Table 2).
Plant feeding damage was assessed visually in each plot using a 0–10 scale following Umina and Hoffmann (2004), where 0 is no visible feeding damage, 5 is approximately 50% damage and 10 represents all plants dead or dying. This method of assessing plant damage has been validated by numerous authors working on H. destructor (e.g., Gillespie 1993; Liu and Ridsdill-Smith 2000; Umina and Hoffmann 2004).
Molecular screening to assess kdr allele frequencies
We used an amplicon sequencing approach to estimate kdr allele frequencies in pooled mite samples at the majority of sampling dates (see Table 2). DNA was extracted from pooled mite samples (average = 41 mites per plot/sampling date; n = 7850 mites in total) using DNeasy Blood and Tissue Kits (Animal Tissue Spin-Column Protocol; Qiagen, Victoria, Australia). Mites were placed into 1.5 ml tubes along with a 3 mm glass bead (Retsch GmbH, Haan, Germany) and 20 μl of ultrapure water. Each tube was then shaken in a Mixer Mill (MM300, Retsch GmbH, Haan, Germany) at 30 oscillations/s for 1 min. After bead beating, 180 μl of ATL buffer and 20 μl of Proteinase K were added to each tube, followed by an incubation step at 56 °C for 1 h. We then followed the Qiagen DNeasy Blood and Tissue Kit protocol for the remaining part of the DNA extraction, with a final elution step of 100 μl AE Buffer added to each sample. Samples were quantified using a Qubit Fluorometer (Invitrogen, Massachusetts, USA) and normalised to 5 ng/μl. We used an amplicon NGS metabarcoding approach following Edwards et al. (2018) with the barcoding approach from Fadrosh et al. (2014). Using primers described in Edwards et al. (2018), we amplified a 313 bp region that included the target mutation using a two-step PCR protocol. The first round PCR primers contained a (5’-3’) universal adaptor sequence and the forward and reverse primers from Edwards et al. (2018). Reaction matrices contained 2 µl DNA, 0.3 µM each primer and 1 × Platinum SuperFi II PCR Master Mix (Thermo Fisher Scientific, Massachusetts, USA) in a final volume of 10 µl. Reaction conditions were as follows: one cycle at 98 °C for 3 min; 40 cycles at 98 °C for 10 s, 54 °C for 10 s, 72 °C for 30 s; and one cycle at 72 °C for 5 min. PCR products were then cleaned with ExoSAP-it (Applied Biosystems, Victoria, Australia).
Second round PCR primers contained the Illumina p5 or p7 binding region (Illumina, California, USA), an 8 bp index sequence (Fadrosh et al. 2014) and a universal adaptor sequence. Unique forward and reverse index combinations were used for each population sample. Reactions contained 2 µl cleaned PCR product, 0.5 µM of each primer and 1 × Platinum SuperFi II PCR Master Mix, in a final volume of 20 µl. Reaction conditions were as follows: one cycle at 98 °C for 1 min; 12 cycles at 98 °C for 10 s, 72 °C for 30 s; and one cycle at 72 °C for 5 min.
Samples were pooled and then subject to size selection to remove non-specific fragments outside of the desired size range, using Sera-Mag SpeedBead Carboxylate-Modified Magnetic Particles (GE Healthcare Life Sciences, Massachusetts, USA). Sequencing was performed on an Illumina MiSeq platform (Illumina, California, USA) using 300 bp PE chemistry, at the Monash Health Translation Precinct (Clayton, Victoria, Australia). Fourteen H2O (PCR negative containing water and no DNA) controls were included in the library.
Bioinformatic analysis of the NGS amplicon data was performed with a custom analysis pipeline that incorporated functions from VSEARCH v2.9.0 (Rognes et al. 2016) and cutadapt (Martin 2011). Forward and reverse reads were merged using fastq_mergepairs, then trimmed to remove primer sequences. Reads were de-replicated and a table was generated detailing the read counts for each sequence in every sample. Since each sample contained a maximum of 50 mites (i.e., 100 alleles), we only included sequences which were present at a frequency of at least 1% of a sample. This resulted in a total of 17 unique sequences (haplotypes). Sequence alignment and analysis of polymorphic sites were carried out using Seaview v4.7 (Gouy et al. 2010) and DnaSP v6 (Rozas et al. 2003). Linkage disequilibrium across all polymorphic sites was assessed using DnaSP v6.
Although the amplicon sequencing approach method is very effective when screening for resistance at a large scale, it becomes less cost efficient when only a few samples are being tested. Additionally, the timeframe required for library preparation and sequencing can be considerable. To overcome these limitations, and to provide further validation of the amplicon sequencing approach used in this study, we developed a droplet digital PCR assay (see Mavridis et al. 2022) that enables kdr allele frequencies to be estimated from pooled samples of H. destructor. The methods we used to develop, and then to validate, the digital PCR assay are detailed in the Supplementary Methods and Figs. S1, S2, S3, S4.
Impact of pyrethroid applications on resistant H. destructor
In addition to examining the fitness costs of resistance in the field, a trial was undertaken to assess the impact of pyrethroid applications on the frequency of pyrethroid resistance in H. destructor. This was achieved by constructing a further 12 exclusion plots at the Tintinara field site. These were established in 2017 and mite populations within each plot were manipulated to create an initial kdr resistance allele frequency of approximately 50% as described above.
Mites within each plot were sprayed with the pyrethroid, Talstar® 250EC (bifenthrin 250 g/L; FMC Australia), at the registered rate for H. destructor, which is 40 ml/ha. Each plot received a spray application on 27 June 2017, immediately after the first sampling date (see Table 1). For six plots, an additional spray of bifenthrin was applied on 18 September 2017. Bifenthrin sprays were applied using an Inter® 16L knapsack (Inter, Victoria, Australia) with a hand-held lance with TeeJet Turbo TwinJet® Twin Flat Spray nozzles (TTJ60-11002). Pressure was maintained at 3 bar and plants were sprayed at a height of 70 cm. Sprays were applied at a controlled speed of ~ 10 km/h to obtain a total spray volume equivalent to 100 L/ha.
At each of the four sampling dates in 2017 (Table 1), mite numbers and plant damage were assessed within each plot following the methodologies described above. Additionally, 100 mites from within each plot were collected at each sampling date and placed into 100% ethanol and frozen at − 20 °C for molecular screening. Approximately 47 mites from each pyrethroid-treated plot at each of the four sampling dates (n = 2281 mites in total) were later genotyped for kdr frequencies using the amplicon sequencing approach.
Statistical analysis
Firstly, we determined if there were any differences between the change in frequency of the two L1024F polymorphisms (TTG/TTC and TTG/TTT alleles; Edwards et al. 2008) over the trial duration. At Arthur River, all alleles were present but there was no statistical evidence that the rate of change was different between the two resistance alleles (χ2 = 1.107, df = 1, p = 0.293) (Fig. S5). Thus, for the remainder of the analyses, the TTC and TTT alleles were pooled together to provide an overall estimate of kdr frequency for Arthur River. At Tintinara, the TTC allele was absent, thus the kdr frequency was estimated based only on the TTT allele at this site.
The measured kdr allele frequency through time t was then modelled using logistic regression with random effects for repeated measures on plots weighted by the number of alleles screened (i.e., twice the number of individual mites):
where \(\alpha\), \(\beta\) and \(\gamma\) are parameters, \(x\) denotes the trial location (Arthur River = 1, Tintinara = 0), \(t\) denotes the normalised time since trial commencement with random effects for each plot \(\epsilon\). Simpler models omitting covariates and more complex models that included interactions did not yield lower AIC scores.
Following this analysis, a population genetics model was developed to estimate fitness costs in terms of standard population genetic parameters, such as relative fitness and dominance. We assumed Hardy–Weinberg ratios after random mating following selection in the previous generation. In the new round of selection in the current generation, the mean fitness of a population \(\overline{w}\) can then be calculated from the proportion of resistance alleles in a population, \(p\) (and susceptible alleles, \(q = 1 {-} p\)), as well as the relative fitness for each genotype \(w_{{{\text{RR}}}}\), \(w_{{{\text{RS}}}}\), and \(w_{{{\text{SS}}}}\):
Assuming the relative fitness of the homozygous susceptible genotype to be \(w_{{{\text{SS}}}} = 1\), the resistance allele frequency in the next generation \(\hat{p}\) can be calculated as:
The dominance of fitness costs \(h\) can be set to determine the relative fitness of heterozygotes as:\(w_{{{\text{RS}}}} = h w_{{{\text{RR}}}} + \left( {1 - h} \right) w_{{{\text{SS}}}}\),with \(h\) = 1, indicating dominance for the resistance allele, and \(h\) = 0, indicating dominance for the recessive allele.
The parameters to vary are therefore \(w_{{{\text{RR}}}}\), \(h\), and the starting allele frequency of resistance at each field site, \(p_{0}\), which can be estimated from data using nonlinear least squares regression. Sampling dates were converted to generation by assuming three evenly spaced generations per year with no development during summer diapause from November to April (see Ridsdill-Smith and Annells 1997).
At Tintinara, the impact of pyrethroid applications on mite abundances between treatment groups was compared using t-tests on log-transformed abundance values. Comparisons were restricted to within each sampling date to ensure independence of data points. Differences in kdr allele frequencies among treatments at each sampling date were analysed using one-way ANOVAs on arcsine square-root transformed data, followed by Tukey-b post hoc tests. Analyses were undertaken in SPSS Statistics v26.
Results
Estimation of fitness costs in the absence of pesticides
Mite abundance and feeding damage
Mite numbers at Arthur River remained relatively consistent across the 4 years (2017–2020) with numbers ranging between 399 and 1338 per m2. At the Tintinara site, there was an initial increase in mite numbers between June and September 2017, followed by a decline in mite numbers over time. In 2019 and 2020, there was a further sharp reduction in mite numbers (compared with 2017 and 2018) at this site (Table 2; Fig. 1). These differences in mite numbers were likely influenced by microclimatic conditions (Solomon 1937; Ridsdill-Smith et al. 2008) and host plant quality (Ridsdill-Smith 1997; Umina and Hoffmann 2004), which were observed to change within field plots over time.
Feeding damage caused by H. destructor was relatively low across both the Arthur River and Tintinara sites (Fig. 2). Arthur River, in particular, experienced minimal damage throughout the trial, with feeding damage scores reaching a maximum of 2.0 within individual plots (in September 2018). Feeding damage at Tintinara from the start of the trial until July 2018 averaged 3.25 across all plots, with a maximum of 6.0 within a single plot observed in July 2018. Feeding damage at Tintinara then decreased in August 2018, and by June 2020, damage scores across all plots averaged 1.0, reflecting the decrease in mite numbers within plots over time (see Fig. 1).
Changes in kdr allele frequency
Of the seventeen unique sequences generated from amplicon sequencing of almost 8000 mites, there were nine polymorphic sites. Analysis of linkage disequilibrium using pairwise comparisons did not reveal any significant correlations between these sites. Polymorphisms at six sites had a frequency of < 0.1% across all samples. Polymorphisms at three sites had a frequency of > 1%, with polymorphisms at two of these sites resulting in non-synonymous changes. One of these sites is L1024F, where previously described polymorphisms known to be associated with pyrethroid resistance in H. destructor have been detected (Edwards et al. 2018). Genotypes at these sites were used in combination to estimate kdr allele frequencies in this study. The other polymorphic site that results in a non-synonymous change is located at F1010V but occurred at a frequency of < 1.5% across all samples. This is a novel polymorphism not previously identified in H. destructor and was not considered in detail in this study. We did consider the frequency of this polymorphism in field plots following pyrethroid applications at the Tintinara field site but saw no evidence for an increase. Given the low overall frequency of the F1010V mutation in the mite populations in this study, further investigations surrounding its role in mediating resistance are warranted.
There was a significant effect of time since trial commencement on kdr allele frequencies measured across sites (χ2 = 167.0, df = 1, p < 0.001), as well as a significant difference of trial site location (χ2 = 68.3, df = 1, p < 0.001). Overall, trial location and time (and their interaction) significantly improved the model compared with simpler models that omitted these variables (Table 3). The estimated coefficient (and 95% confidence intervals) for the effect of the Tintinara site (− 0.68 [− 0.84. − 0.52]) was significantly less than that of the Arthur River site (1.64 [1.47. 1.81]), due to the lower initial frequency of resistance at Tintinara (Fig. 3). The estimated coefficient for time was significantly negative at − 0.18 (− 0.22. − 0.14) and − 0.29 (− 0.35. − 0.23) for Tintinara and Arthur River, respectively, indicating a decreasing frequency of kdr alleles in H. destructor populations through time (Fig. 3). Taken together, these results show that in the absence of pesticide usage, moderate kdr resistance frequencies decrease significantly with time at a rate that differed between sites with a different incidence of resistance.
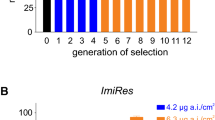
To further estimate the fitness costs of resistance, when only considering changes in kdr resistance allele frequencies, we applied a population genetics model to the data (Table 4). Estimates (and 95% confidence intervals) for the starting frequency of resistance alleles at Arthur River and Tintinara were \(p_{0}^{{\text{A}}}\) = 0.85 (0.82, 0.89) and \(p_{0}^{{\text{T}}}\) = 0.39 (0.35, 0.44), respectively. The relative fitness cost of homozygous resistant individuals was estimated with a high degree of confidence at \(w_{{{\text{RR}}}}\) = 0.86 (0.79, 0.92). This suggests that homozygous resistant individuals are on average ~ 14% less fit than homozygous susceptible individuals. In contrast, the dominance of fitness costs was estimated with less confidence at h = 0.41 (− 0.25. 0.91). This suggests that fitness costs are most likely intermediately dominant. As shown in Fig. 4, the relationship between time and resistance with the best fit was relatively insensitive to other levels of dominance, which accounts for the uncertainty in fitness cost dominance estimation.
Frequency of kdr resistance alleles across estimated generations of H. destructor. Fitted population genetic model predictions estimated with nonlinear least squares are shown by lines for dominant (h = 1), best-fit intermediate (h = 0.4), and recessive (h = 0) fitness costs for the resistance alleles. Points represent means for each sampling date with standard errors shown by vertical bars
Impact of pyrethroid applications on resistant mites
The application of pyrethroids to field plots containing resistant mites at Tintinara in 2017 highlights the strong selection pressure that is exerted when H. destructor are exposed to pesticides from this MoA group (Fig. 5). There was an initial decline in the number of H. destructor immediately following the first chemical treatment, with a significant difference in (log-transformed) mite numbers between the untreated controls and both the bifenthrin × 1 (t = − 6.166, df = 16, p < 0.001) and the bifenthrin × 2 (t = − 6.226, df = 16, p < 0.001) treatments at the August sampling date. Additionally, there was no significant difference in mite numbers between the two bifenthrin treatments (t = 0.052, df = 10, p = 0.959). By September, mite numbers had rebounded, with the bifenthrin × 1 and bifenthrin × 2 treatments surpassing densities within the untreated controls, although these numbers were not significantly different in two-tailed tests (t = 1.997, df = 16, p = 0.059 and t = 1.332, df = 16, p = 0.197, respectively) (Fig. 5a). There was no reduction in mite numbers following the second chemical application undertaken in September 2017, highlighting that the pyrethroid treatments had become ineffective in controlling H. destructor.
Average (a) H. destructor numbers and (b) frequency of kdr resistance alleles in unsprayed and pyrethroid-treated field plots at Tintinara in 2017. Vertical bars represent standard errors and lowercase letters above bars indicate significantly different frequencies within each sampling date (p < 0.05, Tukey-b post hoc tests). The blue and red arrows indicate the timing of the fist chemical application and second chemical application of bifenthrin, respectively
The frequency of kdr resistance alleles across the 12 field plots was ~ 45% at the beginning of 2017 but increased sharply to almost 100% immediately following the first bifenthrin application (Fig. 5b). It remained ~ 95% across all pyrethroid-treated plots thereafter, regardless of whether one or two chemical treatments had been applied. At the August, September and October sampling dates, the frequency of kdr resistance alleles was significantly lower in the untreated control plots compared with the bifenthrin × 1 and bifenthrin × 2 treatments (Fig. 5b).
Discussion
Elucidating fitness costs associated with pesticide resistance in arthropods is not straightforward (Ffrench-Constant and Bass 2017) yet understanding these costs is important when devising strategies to mitigate or manage resistance (Denholm and Rowland 1992). In this study, we identified resistance costs associated with pyrethroid resistance in the pest mite, H. destructor, by showing reductions in the frequency of kdr resistance alleles in the absence of pesticides in two different field populations. Costs associated with kdr-mediated pyrethroid resistance have been suggested previously in H. destructor under shade-house -conditions based on reductions in resistance allele frequencies over time, as well as lower mite numbers across generations in populations with a different incidence of resistance (Cheng et al. 2021). Fitness costs associated with pyrethroid resistance are well documented in a small number of mite pests and many insects (including mosquitoes, flies, moths and aphids), with traits such as development time, overwintering survival, fertility, fecundity, wing surface area and alarm pheromone responses being negatively affected (Foster et al. 1996, 2003, 2005; Kliot and Ghanim 2012; Brito et al. 2013; Rinkevich et al. 2013b; Freeman et al. 2021). Mutations in the target site of pyrethroids, the voltage-gated sodium channel, have been found to reduce overall fitness via a decrease in the efficiency of sodium channel function (Zhao et al. 2000; Heim et al. 2018) and through ‘genetic hitchhiking’ of less fit traits linked to these mutations (Van Straalen and Timmermans 2002; Heim et al. 2018).
The overall fitness of pesticide resistance alleles is dependent on the environment (Hardstone et al. 2009) and, importantly, our study examined the costs of resistance directly under field rather than laboratory or shade-house conditions. Fitness costs associated with resistance alleles can be strongly influenced by the local conditions and seasonal changes (Guillemaud et al. 2003; Rinkevich et al. 2013b; Lee et al. 2020), as well as being affected by different life stages of the organism (Brito et al. 2013). This includes costs associated with kdr. For instance in the house fly, Musca domestica, reductions in the frequency of the kdr resistance mutation, L1014F, occur during winter in regions with a cold climate, but frequencies remain unchanged in regions with warmer climates (Rinkevich et al. 2007). Our study covers these potential sources of variability and meets many of the conditions that should ideally be followed when characterising fitness costs of resistance (Ffrench-Constant and Bass 2017). This includes the ability to test for parallel changes in two geographically separated (by more than 2000 km) populations of H. destructor. Recent high-resolution SNP based data demonstrates kdr resistance has evolved on multiple occasions in H. destructor, with the two populations tested here almost certainly representing independent instances of resistance evolution (Yang et al. 2020). Within each trial site, we established field enclosures with a mixture of resistant and susceptible mites from the same area in order to minimise any confounding effects of genetic backgrounds. Our study also covered multiple years and included all life stages of mites, including the active period during cooler conditions and the intervening summer diapause period (Ridsdill-Smith 1997).
The relative fitness of homozygous resistant (RR) individuals estimated in this study was 0.86 relative to susceptible homozygotes (SS). In other words, kdr-mediated resistance in H. destructor is associated with a fitness cost of ~ 14% in the absence of pesticides. The dominance of this fitness cost was calculated to be 0.40, although there were large confidence intervals around this estimate. It is therefore unclear how much fitness costs in the heterozygote constrain the initial development of resistance, although it is probably sufficient to enable resistance alleles to persist at a very low frequency under field conditions in the absence of persistent strong selection pressure. For instance, if we assume a mutation rate of susceptible to resistance alleles of 10−6, then with a cost to the heterozygote of 0.056 (0.14 × 0.4) we would expect an approximate equilibrium frequency (q) of u ÷ hs (Crow 1970) or 10−6 ÷ 0.056, approximately 1 in 56,000. This means that the RR genotype, which has a high level of phenotypic resistance, would be extremely rare in such a population (1 in 3 billion). On the other hand, once resistance occurs at an appreciable frequency in a H. destructor population, the empirical data collected in our trial involving pesticide sprays suggest resistance alleles will increase in frequency rapidly after only a single pyrethroid application. This trial also allows us to estimate the approximate selective advantage of resistant genotypes based on the sudden increase in kdr allele frequency following chemical treatment. Assuming genotypes were in Hardy–Weinberg equilibrium at the beginning of the trial, when the resistance allele was at a proportion of 0.45 in the plots, the expected frequency of the RR, RS and SS genotypes would have been 0.2025, 0.495 and 0.3025, respectively. After chemical application, the frequency of the resistance allele increased to approximately 0.95, which suggests a high fitness for the RR homozygotes relative to the other two genotypes. Again, assuming Hardy–Weinberg, the expected frequency of the three genotypes after selection and a generation of random mating would have been 0.9025, 0.095 and 0.0025 for RR, RS and SS, respectively. If RS and SS individuals have a similar level of resistance, this change would mean the RS and SS genotypes have a fitness of 0.027 relative to a fitness of 1 for the RR homozygotes (based on the increase in frequency of RR and combined decrease of RS and SS). Earlier studies on H. destructor have shown the RS heterozygote is slightly more resistant than the SS homozygote (Cheng et al. 2019, 2021) and therefore is expected to have a higher fitness than SS individuals when exposed to pyrethroid pesticides. Nonetheless, this does not change the fact that the substantial increase in kdr resistance allele frequency observed in our study requires the RR homozygote to be substantially fitter than the RS heterozygote (as well as the SS genotype).
Combined, our results suggest the efficiency of pyrethroid chemicals for the control of H. destructor may be substantially compromised in Australia into the future. Once established in populations, pyrethroid resistance in H. destructor does not appear to be easily lost, even after several years, emphasizing the need to limit the evolution of resistance in the first place. This potentially explains why pyrethroid resistance in H. destructor continues to persist at very high frequencies in the field and is expanding across Australia (Arthur et al. 2021) despite the existence of fitness costs (although it is important to note that our trials used field exclusion plots that precluded the immigration of mites). Additionally, continued selection from pyrethroids could lead to further genetic changes that alleviate the deleterious effects caused by the initial kdr mutations so that there are no longer fitness costs in resistant mites (Kliot and Ghanim 2012). Such processes have been documented in the sheep blowfly, Lucilia cuprina, where continued use of pesticides after the evolution of resistance eliminated fitness costs through the evolution of a modifier (McKenzie et al. 1982; McKenzie and Clarke 1988).
The physiological basis of the fitness costs quantified in this study remain unknown, and will be difficult to determine given the inherent challenges establishing crosses and culturing large numbers of H. destructor (Ridsdill-Smith 1991). Difficulties establishing mite crosses also means it will be challenging to accurately compare life table parameters and life history traits between resistant and susceptible lines. Genetic alterations due to pesticide resistance generally have pleiotropic effects on multiple traits, causing morphological or behavioural changes that can impair a resistant individuals’ fitness (Kliot and Ghanim 2012). These can manifest in a variety of ways, such as lower reproductive output (Brito et al. 2013; Banazeer et al. 2020), altered development time (Boivin et al. 2001; Brito et al. 2013; Ullah et al. 2020), decreased overwintering survival (Foster et al. 1996; Gazave et al. 2001) and increased vulnerability to predators (Foster et al. 2005). It has been suggested that L1024F may impact the sensitivity of H. destructor to environmental conditions, given crucial summer diapause responses in this species are induced by several environmental cues including daylength, temperature and soil moisture (Cheng et al. 2018, 2019). However, we observed no noticeable shift in either mite numbers or resistance allele frequencies in post-diapause generations (across multiple years) at either the Tintinara or Arthur River site.
The findings from this study have important implications for resistant management programs targeting H. destructor. A key recommendation of the current resistance management strategy developed for this species in Australia (Umina et al. 2018) is the careful rotation of chemical MoA groups across consecutive mite generations. The success of this approach in mitigating resistance issues is predicated by the existence of fitness costs in mites that possess resistance. In theory, the frequency of resistance alleles within a population will decline when another chemical group is used (Ffrench-Constant and Bass 2017). If fitness costs are high, resistance is likely to spread much slower, allowing more opportunities to manage resistance. However, if costs are very low or do not exist, resistance is likely to spread more rapidly and can render resistant management strategies, which rely heavily on chemical rotations, ineffective over the long term (Kliot and Ghanim 2012; Ffrench-Constant and Bass 2017). Additionally, we demonstrate that even with the existence of fitness costs, pyrethroid applications can rapidly increase the frequency of field resistance and render chemicals ineffective against H. destructor. In part, this is due to the very high level of pyrethroid resistance conferred by kdr in H. destructor (> 200,000 to bifenthrin), which has been demonstrated through laboratory bioassays, including on populations collected from the Tintinara field site used in this study (see Umina 2007; Maino et al. 2018, 2021; Edwards et al. 2018). Thus, the gradual reversal to susceptibility within a mite population that would be expected after eliminating pesticides can be quickly undone by a single pyrethroid spray. This highlights the importance of adopting other management strategies to reduce the impacts of resistance in these mites such as strip spraying to allow for ongoing immigration of susceptible mites (Maino et al. 2021). The development of a high throughput digital PCR approach to detect kdr resistance should further assist in the ongoing management of H. destructor by allowing small mite numbers to be cost-effectively and quickly screened for kdr resistance. Because of this, resistance surveillance results can be relayed to farmers in a short space of time and on-farm management strategies adjusted where required.
Data availability
The datasets generated in this study are available from the corresponding author on reasonable request.
References
Annells AJ, Ridsdill-Smith TJ (1994) Host plant species and carbohydrate supplements affecting rate of multiplication of redlegged earth mite. Exp Appl Acarol 18:521–530. https://doi.org/10.1007/BF00058935
Arthur AL, Maino J, Hoffmann AA et al (2021) Learnings from over a decade of increasing pesticide resistance in the redlegged earth mite, Halotydeus destructor (Tucker). Pest Manag Sci 77:3013–3024. https://doi.org/10.1002/ps.6340
Banazeer A, Shad SA, Shahzad Afzal MB (2020) Laboratory induced bifenthrin resistance selection in Oxycarenus hyalinipennis (Costa) (Hemiptera: Lygaeidae): stability, cross-resistance, dominance and effects on biological fitness. Crop Prot 132:105107. https://doi.org/10.1016/j.cropro.2020.105107
Boivin T, Chabert D’Hières C, Bouvier JC et al (2001) Pleiotropy of insecticide resistance in the codling moth, Cydia pomonella. Entomol Exp Appl 99:381–386. https://doi.org/10.1046/j.1570-7458.2001.00838.x
Brito LP, Linss JGB, Lima-Camara TN et al (2013) Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS One. https://doi.org/10.1371/journal.pone.0060878
Cheng X, Hoffmann AA, Maino JL, Umina PA (2018) A cryptic diapause strategy in Halotydeus destructor (Tucker) (Trombidiformes: Penthaleidae) induced by multiple cues. Pest Manag Sci 74:2618–2625. https://doi.org/10.1002/ps.5053
Cheng X, Umina PA, Lee SF, Hoffmann AA (2019) Pyrethroid resistance in the pest mite, Halotydeus destructor: dominance patterns and a new method for resistance screening. Pestic Biochem Physiol 159:9–16. https://doi.org/10.1016/j.pestbp.2019.04.010
Cheng X, Hoffmann AA, Edwards OR, Umina PA (2021) Fitness costs associated with pyrethroid resistance in Halotydeus destructor (Tucker) (Acari: Penthaleidae) elucidated through semi-field trials. J Econ Entomol 114:1270–1281. https://doi.org/10.1093/jee/toab031
Crow JF (1970) Genetic loads and the cost of natural selection. In: Kojima K (ed) Mathematical topics in population genetics. Biomathematics, vol 1. Springer, Germany
Denholm I, Rowland MW (1992) Tactics for managing pesticide resistance in arthropods: theory and practice. Annu Rev Entomol 37:91–112. https://doi.org/10.1146/annurev.en.37.010192.000515
Edwards OR, Walsh TK, Metcalfe S et al (2018) A genomic approach to identify and monitor a novel pyrethroid resistance mutation in the redlegged earth mite, Halotydeus destructor. Pestic Biochem Physiol 144:83–90. https://doi.org/10.1016/j.pestbp.2017.12.002
Fadrosh DW, Ma B, Gajer P et al (2014) An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6. https://doi.org/10.1186/2049-2618-2-6
Ffrench-Constant RH, Bass C (2017) Does resistance really carry a fitness cost? Curr Opin Insect Sci 21:39–46. https://doi.org/10.1016/j.cois.2017.04.011
Foster SP, Harrington R, Devonshire AL et al (1996) Comparative survival of insecticide-susceptible and resistant peach–potato aphids, Myzus persicae (Sulzer) (Hemiptera: Aphididae), in low temperature field trials. Bull Entomol Res 86:17–27. https://doi.org/10.1017/s0007485300052159
Foster SP, Young S, Williamson MS et al (2003) Analogous pleiotropic effects of insecticide resistance genotypes in peach–potato aphids and houseflies. Heredity (Edinb) 91:98–106. https://doi.org/10.1038/sj.hdy.6800285
Foster SP, Denholm I, Thompson R et al (2005) Reduced response of insecticide-resistant aphids and attraction of parasitoids to aphid alarm pheromone; a potential fitness trade-off. Bull Entomol Res 95:37–46. https://doi.org/10.1079/ber2004336
Freeman JC, San Miguel K, Scott JG (2021) All resistance alleles are not equal: the high fitness cost of super-kdr in the absence of insecticide. Pest Manag Sci 77:3693–3697. https://doi.org/10.1002/ps.6115
Gazave E, Chevillon C, Lenormand T et al (2001) Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity (Edinb) 87:441–448. https://doi.org/10.1046/j.1365-2540.2001.00926.x
Gillespie DJ (1991) Identification of resistance to redlegged earth mite (Halotydeus destructor) in pasture legumes. Plant Prot Q 6:170–171
Gouy M, Guindon S, Gascuel O (2010) SeaView Version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27:221–224. https://doi.org/10.1093/molbev/msp259
Guillemaud T, Brun A, Anthony N et al (2003) Incidence of insecticide resistance alleles in sexually-reproducing populations of the peach–potato aphid Myzus persicae (Hemiptera: Aphididae) from southern France. Bull Entomol Res 93:289–297. https://doi.org/10.1079/ber2003241
Hardstone MC, Lazzaro BP, Scott JG (2009) The effect of three environmental conditions on the fitness of cytochrome P450 monooxygenase-mediated permethrin resistance in Culex pipiens quinquefasciatus. BMC Evol Biol 9:42. https://doi.org/10.1186/1471-2148-9-42
Heim JR, Weston DP, Major K et al (2018) Are there fitness costs of adaptive pyrethroid resistance in the amphipod, Hyalella azteca? Environ Pollut 235:39–46. https://doi.org/10.1016/j.envpol.2017.12.043
Kliot A, Ghanim M (2012) Fitness costs associated with insecticide resistance. Pest Manag Sci 68:1431–1437. https://doi.org/10.1002/ps.3395
Lee DE, Kim HC, Chong ST et al (2020) Regional and seasonal detection of resistance mutation frequencies in field populations of Anopheles Hyrcanus Group and Culex pipiens complex in Korea. Pestic Biochem Physiol 164:33–39. https://doi.org/10.1016/j.pestbp.2019.12.005
Liu A, Ridsdill-Smith TJ (2000) Feeding by redlegged earth mite (Halotydeus destructor) on seedlings influences subsequent plant performance of different pulse crops. Aust J Exp Agric 40:715–723. https://doi.org/10.1111/j.1440-6055.1991.tb00443.x
Maino JL, Binns M, Umina P (2018) No longer a west-side story—pesticide resistance discovered in the eastern range of a major Australian crop pest, Halotydeus destructor (Acari: Penthaleidae). Crop Pasture Sci 69:216–221. https://doi.org/10.1071/CP17327
Maino JL, Hoffmann AA, Binns M et al (2021) Strip spraying delays pyrethroid resistance in the redlegged earth mite, Halotydeus destructor: a novel refuge strategy. Pest Manag Sci 77:4572–4582. https://doi.org/10.1002/ps.6497
Martin M (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. https://doi.org/10.14806/ej.17.1.200
Mavridis K, Papapostolou K, Riga M et al (2022) Multiple TaqMan qPCR and droplet digital PCR (ddPCR) diagnostics for pesticide resistance monitoring and management, in the major agricultural pest Tetranychus urticae. Pest Manag Sci 78:263–273. https://doi.org/10.1002/ps.6632
McDonald G, Umina PA, Macfadyen S et al (2015) Predicting the timing of first generation egg hatch for the pest redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Exp Appl Acarol 65:259–276. https://doi.org/10.1007/s10493-014-9876-x
McKenzie JA, Clarke GM (1988) Diazinon resistance, fluctuating asymmetry and fitness in the Australian sheep blowfly, Lucilia cuprina. Genetics 120:213–220. https://doi.org/10.1093/genetics/120.1.213
McKenzie JA, Whitten MJ, Adena MA (1982) The effect of genetic background on the fitness of diazinon resistance genotypes of the Australian sheep blowfly, Lucilia cuprina. Heredity 49:1–9. https://doi.org/10.1038/hdy.1982.60
Ridsdill-Smith TJ (1991) Laboratory rearing of Halotydeus destructor (Tucker) (Acari: Penthaleidae). Aust J Entomol 30:313. https://doi.org/10.1111/j.1440-6055.1991.tb00443.x
Ridsdill-Smith TJ (1997) Biology and control of Halotydeus destructor (Tucker) (Acarina: Penthaleidae): a review. Exp Appl Acarol 21:195–224. https://doi.org/10.1023/A:1018490703897
Ridsdill-Smith TJ, Annells AJ (1997) Seasonal occurrence and abundance of redlegged earth mite Halotydeus destructor (Acari: Penthaleidae) in annual pastures of southwestern Australia. Bull Entomol Res 87:413–423. https://doi.org/10.1017/S0007485300037421
Ridsdill-Smith TJ, Hoffmann AA, Mangano GP et al (2008) Strategies for control of the redlegged earth mite in Australia. Aust J Exp Agric 48:1506. https://doi.org/10.1071/EA08020
Rinkevich FD, Hamm RL, Geden CJ, Scott JG (2007) Dynamics of insecticide resistance alleles in house fly populations from New York and Florida. Insect Biochem Mol Biol 37:550–558. https://doi.org/10.1016/j.ibmb.2007.02.013
Rinkevich FD, Du Y, Dong K (2013a) Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol 106:93–100. https://doi.org/10.1016/j.pestbp.2013.02.007
Rinkevich FD, Leichter CA, Lazo TA et al (2013b) Variable fitness costs for pyrethroid resistance alleles in the house fly, Musca domestica, in the absence of insecticide pressure. Pestic Biochem Physiol 105:161–168. https://doi.org/10.1016/j.pestbp.2013.01.006
Robinson MT, Hoffmann AA (2001) The pest status and distribution of three cryptic blue oat mite species (Penthaleus spp.) and redlegged earth mite (Halotydeus destructor) in southeastern Australia. Exp Appl Acarol 25:699–716. https://doi.org/10.1023/A:1016375219330
Rognes T, Flouri T, Nichols B et al (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584. https://doi.org/10.7717/peerj.2584
Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497. https://doi.org/10.1093/bioinformatics/btg359
Soderlund DM (2008) Pyrethroids, knockdown resistance and sodium channels. Pest Manag Sci 64:610–616. https://doi.org/10.1002/ps.1574
Solomon ME (1937) Experiments on the effects of temperature and humidity on the survivial of Halotydues destructor (Tucker), Acarina fam. Penthaleidae. Aust J Exp Biol Med Sci 15:1–16. https://doi.org/10.1038/icb.1937.1
Ullah F, Gul H, Tariq K et al (2020) Fitness costs in clothianidin-resistant population of the melon aphid Aphis gossypii. PLoS One 15:e0238707. https://doi.org/10.1371/journal.pone.0238707
Umina PA (2007) Pyrethroid resistance discovered in a major agricultural pest in southern Australia: the redlegged earth mite Halotydeus destructor (Acari: Penthaleidae). Pest Manag Sci 63:1185–1190. https://doi.org/10.1002/ps.1439
Umina PA, Hoffmann AA (2003) Diapause and implications for control of Penthaleus species and Halotydeus destructor (Acari: Penthaleidae) in southeastern Australia. Exp Appl Acarol 31:209–223. https://doi.org/10.1023/B:APPA.0000010378.91111.16
Umina PA, Hoffmann AA (2004) Plant host associations of Penthaleus species and Halotydeus destructor (Acari: Penthaleidae) and implications for integrated pest management. Exp Appl Acarol 33:1–20. https://doi.org/10.1023/B:APPA.0000030014.00162.44
Umina PA, Hoffmann AA (2005) Competitive interactions among four pest species of earth mites (Acari: Penthaleidae). J Econ Entomol 98:307–316. https://doi.org/10.1093/jee/98.2.307
Umina PA, Weeks AR, Roberts J et al (2012) The current status of pesticide resistance in Australian populations of the redlegged earth mite (Halotydeus destructor). Pest Manag Sci 68:889–896. https://doi.org/10.1002/ps.3247
Umina PA, Lord A, Micic S, Edwards O (2017) Discovery and characterisation of field resistance to organophosphorus chemicals in a major mite pest, Halotydeus destructor. Pest Manag Sci 73:1719–1724. https://doi.org/10.1002/ps.4520
Umina PA, Hoffmann AA, McDonald G et al (2018) Resistance management strategy for the redlegged earth mite in Australian grains and pastures. Grains Research and Development Corporation, Canberra, Australia
Umina PA, McDonald G, Maino J et al (2019) Escalating insecticide resistance in Australian grain pests: contributing factors, industry trends and management opportunities. Pest Manag Sci 75:1494–1506. https://doi.org/10.1002/ps.5285
Van Straalen NM, Timmermans MJTN (2002) Genetic variation in toxicant-stressed populations: an evaluation of the “genetic erosion” hypothesis. Hum Ecol Risk Assess 8:983–1002. https://doi.org/10.1080/1080-700291905783
Weeks AR, Hoffmann AA (2000) Competitive interactions between two pest species of earth mites, Halotydeus destructor and Penthaleus major (Acarina: Penthaleidae). J Econ Entomol 93:1183–1191. https://doi.org/10.1603/0022-0493-93.4.1183
Weeks AR, Fripp YJ, Hoffmann AA (1995) Genetic structure of Halotydeus destructor and Penthaleus major populations in Victoria (Acari: Penthaleidae). Exp Appl Acarol 19:633–646. https://doi.org/10.1007/BF00145252
Yang Q, Umina PA, Rašić G et al (2020) Origin of resistance to pyrethroids in the redlegged earth mite (Halotydeus destructor) in Australia: repeated local evolution and migration. Pest Manag Sci 76:509–519. https://doi.org/10.1002/ps.5538
Zhao Y, Park Y, Adams ME (2000) Functional and evolutionary consequences of pyrethroid resistance mutations in S6 transmembrane segments of a voltage-gated sodium channel. Biochem Biophys Res Commun 278:516–521. https://doi.org/10.1006/bbrc.2000.3832
Acknowledgements
We thank Amol Ghodke for technical assistance and the growers who generously allowed us to undertake these trials on their properties.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported through funding from the Grains Research and Development Corporation (Grant No. UM00057).
Author information
Authors and Affiliations
Contributions
This experiment was conceived and designed by PU, JM, AH and OE. Data were collected by OE, MB, JM and XC. Molecular analyses were performed by AvR and SVS, and JM conducted the statistical analysis. Data were interpreted by JM, PU, AW and AH. The manuscript was drafted by PU, AH, JM, OR and AA, with input from all authors. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no financial or non-financial conflict of interest.
Additional information
Communicated by Maohua Chen.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umina, P.A., Maino, J.L., Edwards, O. et al. Fitness costs of pyrethroid resistance in the polyphagous pest mite, Halotydeus destructor, under field conditions. J Pest Sci 97, 185–197 (2024). https://doi.org/10.1007/s10340-023-01605-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01605-9