Abstract
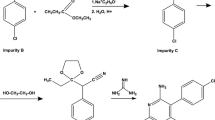
Ivermectin injectable product is widely used as a parasiticide for the treatment and control of internal and external parasites of cattle and swine. A reversed-phase high performance liquid chromatography (RP-HPLC) method has been developed for the assay of ivermectin, including the determination and identification of its related substances in an ivermectin injectable product. Ivermectin and its related substances are adequately separated using a gradient elution at a flow rate of 1.5 mL/min on a Zorbax Extend-C18 column (150 × 4.6 mm i.d., 3.5 µm particle size) maintained at 30 °C. Mobile phase-A is composed of water and mobile phase-B is composed of acetonitrile/methanol (85/15, v/v). Analytes were monitored by UV detection at 245 nm. The stability-indicating capability of the method has been demonstrated by adequately separating the degradation products from the stress degraded samples of the ivermectin injectable product. The analytical performance of the method was validated as per the current ICH guidelines with respect to specificity, linearity (R2 > 0.999), accuracy, precision, detection limit (0.3 µg/mL), quantitation limit (1.0 µg/mL), and robustness. This newly developed HPLC method for assay of ivermectin and determination of its related substances can be used to increase the throughput of a quality control (QC) lab for analytical testing of ivermectin injectable products.



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Campbell WC (1985) Ivermectin: an update. Parasitol Today 1:10–16. https://doi.org/10.1016/0169-4758(85)90100-0
Geary TG (2005) Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol 21:530–532. https://doi.org/10.1016/j.pt.2005.08.014
Õmura S, Crump A (2004) The life and times of ivermectin—a success story. Nat Rev Microbiol 2:984–989. https://doi.org/10.1038/nrmicro1048
Vercruysse J, Rew R (2002) Macrocyclic lactones in antiparasitic therapy. CABI Publishing, Wallingford, UK
Martin RJ, Robertson AP, Choudhary S (2020) Ivermectin: an anthelmintic, an insecticide, and much more. Trends Parasitol 37:48–64. https://doi.org/10.1016/j.pt.2020.10.005
Mastrangelo E, Pezzullo M, Burghgraeve TD et al (2012) Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemoth 67:1884–1894. https://doi.org/10.1093/jac/dks147
Wagstaff KM, Sivakumaran H, Heaton SM et al (2012) Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J 443:851–856. https://doi.org/10.1042/bj20120150
Varghese FS, Kaukinen P, Gläsker S et al (2016) Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir Res 126:117–124. https://doi.org/10.1016/j.antiviral.2015.12.012
Heidary F, Gharebaghi R (2020) Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiotics 73:593–602. https://doi.org/10.1038/s41429-020-0336-z
Jenke D (2002) Extractable/leachable substances from plastic materials used as pharmaceutical product containers/devices. Pda J Pharm Sci Technology Pda 56:332–371
Cuadros-Rodríguez L, Lazúen-Muros M, Ruiz-Samblás C, Navas-Iglesias N (2020) Leachables from plastic materials in contact with drugs. State of the art and review of current analytical approaches. Int J Pharmaceut 583:119332. https://doi.org/10.1016/j.ijpharm.2020.119332
Abend AM, Chung L, McCollum DG, Wuelfing WP (2003) Development and validation of an automated extraction method (accelerated solvent extraction®) and a reverse-phase HPLC analysis method for assay of ivermectin in a meat-based chewable formulation. J Pharmaceut Biomed 31:1177–1183. https://doi.org/10.1016/s0731-7085(03)00020-7
Kitzman D, Wei S-Y, Fleckenstein L (2006) Liquid chromatographic assay of ivermectin in human plasma for application to clinical pharmacokinetic studies. J Pharmaceut Biomed 40:1013–1020. https://doi.org/10.1016/j.jpba.2005.08.026
Shurbaji M, Rub MHAA, Saket MM et al (2010) Development and validation of a new HPLC-UV method for the simultaneous determination of triclabendazole and ivermectin B1a in a pharmaceutical formulation. J Aoac Int 93:1868–1873
Saad AS, Ismail NS, Soliman M, Zaazaa HE (2016) Validated stability-indicating RP-HPLC method for simultaneous determination of Clorsulon and Ivermectin employing Plackett-Burman experimental design for robustness testing. J Aoac Int 99:571–578. https://doi.org/10.5740/jaoacint.15-0128
Devaka NVSK, Rao VM (2019) Chromatographic quantification of Ivermectin and Pranziquantel in the tablets using stability indicating RP-HPLC method. Pharm Sci 25:254–261
Mohd A, Alam S, Ahmad S et al (2011) Determination of ivermectin stability by high-performance thin-layer chromatography. Int J Drug Dev & Res 2:240–247
Wani GP, Jadhav SB (2018) RP-HPLC and HPTLC stability indicating assay methods for ivermectin in bulk and tablet dosage form. Indian Drugs 3:33–42
Chhonker YS, Ma L, Edi C, Murry DJ (2018) A sensitive and selective LCMS/MS method for quantitation of ivermectin in human, mouse and monkey plasma: clinical validation. Bioanalysis 10:1841–1852. https://doi.org/10.4155/bio-2018-0110
Ortiz AJ, Cortez V, Azzouz A, Verdú JR (2017) Isolation and determination of ivermectin in post-mortem and in vivo tissues of dung beetles using a continuous solid phase extraction method followed by LC-ESI+-MS/MS. PLoS ONE 12:e0172202. https://doi.org/10.1371/journal.pone.0172202
Croubels S, Baere SD, Cherlet M, Backer PD (2002) Determination of ivermectin B1a in animal plasma by liquid chromatography combined with electrospray ionization mass spectrometry. J Mass Spectrom 37:840–847. https://doi.org/10.1002/jms.343
European Pharmacopoeia 10th edn (10.6). Ivermectin. Accessed 8 Aug 2021
United States Pharmacopeia, Ivermectin Injection. Accessed 19 May 2021
ICH Q2(R1) (2005) guidelines, http://www.ich.org/page/quality-guidelines. Accessed 19 May 2021
Acknowledgements
The authors would like to thank all the analytical scientists in BIAH—Global Pharmaceutical Technical Support (GPTS) group for their support of this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
This document is provided for scientific purpose only. Any reference to a brand or trademark herein is for informational purpose only and is not intended for a commercial purpose or to dilute the rights of the respective owner(s) of the brand(s) or trademark(s).
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
The authors’ affiliated company, Boehringer Ingelheim Animal Health USA Inc. approved the submission of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Padivitage, N., Adhikari, S. & Rustum, A.M. Assay of Ivermectin Including Determination and Identification of Its Related Substances in Ivermectin Injectable Product by a Stability-Indicating RP-HPLC Method. Chromatographia 84, 989–997 (2021). https://doi.org/10.1007/s10337-021-04088-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04088-x