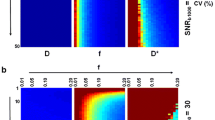
Abstract
Object
We aimed to modify our previously published method for arterial input function measurements for evaluation of cerebral perfusion (dynamic susceptibility contrast MRI) such that it can be applied in humans in a clinical setting.
Materials and methods
Similarly to our previous work, a conventional measurement sequence for dynamic susceptibility contrast MRI is extended with an additional measurement slice at the neck. Measurement parameters at this slice were optimized for the blood signal (short echo time, background suppression, magnitude and phase images). Phase-based evaluation of the signal in the carotid arteries is used to obtain quantitative arterial input functions.
Results
In all pilot measurements, quantitative arterial input functions were obtained. The resulting absolute perfusion parameters agree well with literature values (gray and white matter mean values of 46 and 24 mL/100 g/min, respectively, for cerebral blood flow and 3.0% and 1.6%, respectively, for cerebral blood volume).
Conclusions
The proposed method has the potential to quantify arterial input functions in the carotid arteries from a direct measurement without any additional normalization.






Similar content being viewed by others
References
Østergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med 36(5):715–725
Østergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn Reson Med 36:726–735
Conturo TE, Barker PB, Mathews VP, Monsein LH, Bryan RN (1992) MR imaging of cerebral perfusion by phase-angle reconstruction of bolus paramagnetic-induced frequency shifts. Magn Reson Med 27(2):375–390
Akbudak E, Conturo TE (1996) Arterial input functions from MR phase imaging. Magn Reson Med 36(6):809–815
van Osch MJ, Vonken EJ, Viergever MA, van der Grond J, Bakker CJ (2003) Measuring the arterial input function with gradient echo sequences. Magn Reson Med 49:1067–1076
Calamante F, Morup M, Hansen LK (2004) Defining a local arterial input function for perfusion MRI using independent component analysis. Magn Reson Med 52(4):789–797
Mouridsen K, Christensen S, Gyldensted L, Ostergaard L (2006) Automatic selection of arterial input function using cluster analysis. Magn Reson Med 55(3):524–531
Kotys MS, Akbudak E, Markham J, Conturo TE (2007) Precision, signal-to-noise ratio, and dose optimization of magnitude and phase arterial input functions in dynamic susceptibility contrast MRI. J Magn Reson Imaging 25(3):598–611
Bleeker EJW, van Buchem MA, van Osch MJP (2009) Optimal location for arterial input function measurements near the middle cerebral artery in first-pass perfusion MRI. J Cereb Blood Flow Metab 29(4):840–852
Kellner E, Mader I, Mix M, Splitthoff DN, Reisert M, Foerster K, Nguyen-Thanh T, Gall P, Kiselev VG (2013) Arterial input function measurements for bolus tracking perfusion imaging in the brain. Magn Reson Med 69(3):771–780
Bleeker EJW, van Buchem MA, Webb AG, van Osch MJP (2010) Phase-based arterial input function measurements for dynamic susceptibility contrast MRI. Magn Reson Med 64(2):358–368
Bernstein MA, King KF, Zhou XJ (2004) Handbook of MRI pulse sequences. Elsevier, Amsterdam
Bruder H, Fischer H, Reinfelder HE, Schmitt F (1992) Image reconstruction for echo planar imaging with nonequidistant k-space sampling. Magn Reson Med 23(2):311–323
van Osch MJP, van der Grond J, Bakker CJG (2005) Partial volume effects on arterial input functions: shape and amplitude distortions and their correction. J Magn Reson Imaging 22(6):704–709
Kellner E, Mix M, Reisert M, Förster K, Nguyen-Thanh T, Splitthoff DN, Gall P, Kiselev VG, Mader I (2014) Quantitative cerebral blood flow with bolus tracking perfusion MRI: measurements in porcine model and comparison with \({{\rm H}}_2{^{15}O}\) PET. Magn Reson Med 72(6):1723–1734
Yablonskiy DA, Haacke EM (1994) Theory of NMR signal behavior in magnetically inhomogeneous tissues: the static dephasing regime. Magn Reson Med 32(6):749–763
Calamante F, Gadian D, Connelly A (2002) Quantification of perfusion using bolus tracking magnetic resonance imaging in stroke: assumptions, limitations, and potential implications for clinical use. Stroke 33(4):1146–51
Lin W, Celik A, Derdeyn C, An H, Lee Y, Videen T, Oestergaard L, Powers WJ (2001) Quantitative measurements of cerebral blood flow in patients with unilateral carotid artery occlusion: a PET and MR study. J Magn Reson Imaging 14(6):659–67
Zaharchuk G, Bammer R, Straka M, Newbould RD, Rosenberg J, Olivot JM, Mlynash M, Lansberg MG, Schwartz NE, Marks MM et al (2009) Improving dynamic susceptibility contrast MRI measurement of quantitative cerebral blood flow using corrections for partial volume and nonlinear contrast relaxivity: a xenon computed tomographic comparative study. J Magn Reson Imaging 30(4):743–752
Knutsson L, van Westen D, Petersen ET, Bloch KM, Holtås S, Ståhlberg F, Wirestam R (2010) Absolute quantification of cerebral blood flow: correlation between dynamic susceptibility contrast MRI and model-free arterial spin labeling. Magn Reson Med 28(1):1–7
Østergaard L, Johannsen P, Høst-Poulsen P, Vestergaard-Poulsen P, Asboe H, Gee AD, Hansen SB, Cold GE, Gjedde A, Gyldensted C (1998) Cerebral blood flow measurements by magnetic resonance imaging bolus tracking: comparison with \([{}^{15}{{\rm O}}]{{\rm H}}_{2}{{\rm O}}\) positron emission tomography in humans. J Cereb Blood Flow Metab 18(9):935–940
van Osch MJP, Vonken EJPA, Wu O, Viergever MA, van der Grond J, Bakker CJG (2003) Model of the human vasculature for studying the influence of contrast injection speed on cerebral perfusion MRI. Magn Reson Med 50(3):614–622
Calamante F (2005) Bolus dispersion issues related to the quantification of perfusion MRI data. J Magn Reson Imaging 22(6):718–722
Mouannes-Srour JJ, Shin W, Ansari SA, Hurley MC, Vakil P, Bendok BR, Lee JL, Derdeyn CP, Carroll TJ (2012) Correction for arterial-tissue delay and dispersion in absolute quantitative cerebral perfusion DSC MR imaging. Magn Reson Med 68(2):495–506
Kellner E, Gall P, Günther M, Reisert M, Mader I, Fleysher R, Kiselev VG (2014) Blood tracer kinetics in the arterial tree. PLoS One 9(10):e109230
Willats L, Connelly A, Calamante F (2006) Improved deconvolution of perfusion MRI data in the presence of bolus delay and dispersion. Magn Reson Med 56(1):146–156
Frackowiak RS, Lenzi GL, Jones T, Heather JD (1980) Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Cardiovasc Comput Tomogr 4(6):727–736
Yamaguchi T, Kanno I, Uemura K, Shishido F, Inugami A, Ogawa T, Murakami M, Suzuki K (1986) Reduction in regional cerebral metabolic rate of oxygen during human aging. Stroke 17(6):1220–1228
Leenders K, Perani D, Lammertsma A, Heather J, Buckingham P, Jones T, Healy M, Gibbs J, Wise R, Hatazawa J et al (1990) Cerebral blood flow, blood volume and oxygen utilization normal values and effect of age. Brain 113(1):27–47
Ito H, Kanno I, Kato C, Sasaki T, Ishii K, Ouchi Y, Iida A, Okazawa H, Hayashida K, Tsuyuguchi N et al (2004) Database of normal human cerebral blood flow, cerebral blood volume, cerebral oxygen extraction fraction and cerebral metabolic rate of oxygen measured by positron emission tomography with 15O-labelled carbon dioxide or water, carbon monoxide and oxygen: a multicentre study in Japan. Eur J Nucl Med Mol Imaging 31(5):635–643
Grandin CB, Bol A, Smith AM, Michel C, Cosnard G (2005) Absolute CBF and CBV measurements by MRI bolus tracking before and after acetazolamide challenge: repeatabilily and comparison with PET in humans. Neuroimage 26(2):525–535
Ibaraki M, Ito H, Shimosegawa E, Toyoshima H, Ishigame K, Takahashi K, Kanno I, Miura S (2006) Cerebral vascular mean transit time in healthy humans: a comparative study with PET and dynamic susceptibility contrast-enhanced MRI. J Cereb Blood Flow Metab 27(2):404–413
Sakaie KE, Shin W, Curtin KR, McCarthy RM, Cashen TA, Carroll TJ (2005) Method for improving the accuracy of quantitative cerebral perfusion imaging. J Magn Reson Imaging 21(5):512–519
Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ (2007) Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med 58(6):1232–1241
Mouannes Srour J, Shin W, Shah S, Sen A, Carroll TJ (2010) SCALE-PWI: a pulse sequence for absolute quantitative cerebral perfusion imaging. J Cereb Blood Flow Metab 31(5):1272–1282
Petersen ET, Mouridsen K, Golay X (2010) The QUASAR reproducibility study, part II: results from a multi-center arterial spin labeling test-retest study. Neuroimage 49(1):104–113
Acknowledgements
We are grateful to Hansjörg Mast. The work was supported by the German Research Council (grants KI-1089/2-1 and MA 2343/4-1).
Author information
Authors and Affiliations
Contributions
Author contributions
EK was responsible for protocol/project development, data collection or management, and data analysis. IM was responsible for protocol/project development and data collection or management. MR was responsible for data analysis. HU was responsible for data collection or management. VGK was responsible for protocol/project development and data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards..
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kellner, E., Mader, I., Reisert, M. et al. Arterial input function in a dedicated slice for cerebral perfusion measurements in humans. Magn Reson Mater Phy 31, 439–448 (2018). https://doi.org/10.1007/s10334-017-0663-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-017-0663-7